Article Text
Abstract
Objectives To evaluate the risk of incident myocardial infarction (MI), stroke and peripheral vascular disease (PVD) in individuals with systemic sclerosis (SSc) in a general population context.
Methods We conducted a cohort study using a UK primary care database containing records from 1986 to 2011. SSc diagnoses, outcomes and cardiovascular risk factors were identified from electronic medical records. We conducted two cohort analyses: (1) MI and stroke, and (2) PVD, excluding individuals with prevalent disease at baseline for each analysis. We estimated HRs comparing SSc with age-, sex- and entry time-matched comparison cohorts, adjusting for potential cardiovascular risk factors.
Results Among 865 individuals with SSc (85.8% women, mean age 58.7 years), the incidence rates (IRs) of MI and stroke were 4.4 and 4.8 per 1000 person-years (PY), versus 2.5 and 2.5 per 1000 PY in the comparison cohort. The corresponding adjusted HRs were 1.80 (95% CI 1.07 to 3.05) for MI and 2.61 (95% CI 1.54 to 4.44) for stroke. Among 858 individuals with SSc (85.3% female, mean age 58.9 years), the IR of PVD was 7.6 per 1000 PY versus 1.9 per 1000 PY in the comparison cohort, with an adjusted HR of 4.35 (95% CI 2.74 to 6.93).
Conclusions These findings provide the first general population-based evidence that SSc is associated with an increased risk of developing MI, stroke and PVD. Further insight into disease mechanisms, as well as how disease subtype, organ involvement and medication use may alter these increased risks, is needed.
- Systemic Sclerosis
- Cardiovascular Disease
- Atherosclerosis
- Epidemiology
Statistics from Altmetric.com
Introduction
An increased risk of premature atherosclerotic disease in patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) has been well described in the recent literature.1 ,2 Much of the association between autoimmune diseases and atherosclerosis is believed to occur through chronic inflammation.3 ,4 Systemic sclerosis (SSc), a related autoimmune disease with a less prominent inflammatory component but significant vascular abnormalities,5 such as Raynaud's phenomenon and pulmonary hypertension, has been proposed to cause premature atherosclerosis as well.6–8
Two meta-analyses of studies assessing subclinical atherosclerosis showed that SSc patients had significantly increased carotid intima-media thickness (IMT), indicating an increased atherosclerotic burden, as compared with controls.9 ,10 This increased mean difference in carotid IMT reported (0.11 mm) was between that of patients with RA (0.09 mm) and diabetes (0.13 mm).9 SSc has also been shown to be an independent risk factor for coronary calcification, another marker of atherosclerosis and predictor of future coronary events.11 ,12 Furthermore, a recent cross-sectional analysis reported an increased prevalence of coronary artery disease among SSc patients (referred from rheumatology practices) as compared with external population-based cohorts.13 However, whether these findings of subclinical atherosclerosis and cross-sectional associations translate to an increased future risk of cardiovascular disease (CVD) among individuals with SSc is unknown.
SSc is currently associated with the highest mortality among all the connective tissue diseases, with an estimated 10 year survival of 66–82%.14–16 With recent improvements in the treatment of renal crisis and pulmonary hypertension, patients with SSc are dying less from SSc related problems and more from non-SSc related causes, which now account for close to 50% of all SSc deaths.14 ,17 As CVD contributes significantly to this mortality burden, accounting for 20–30% of all SSc deaths,8 an accurate understanding of CVD risk is crucial to improving the overall outcomes of SSc. Therefore, to address these issues, we evaluated the future risk of developing incident myocardial infarctions (MIs), strokes and peripheral vascular disease (PVD) in individuals with SSc in a general population context.
Methods
Data source
The Health Improvement Network (THIN) is an electronic medical record database derived from general practices in the UK. Data on approximately 7.3 million patients are recorded by general practitioners (GPs) as part of patients’ clinical records and sent anonymously to THIN. Since approximately 97% of the UK population is registered with a GP,18 THIN data are representative of the UK general population.19 THIN data include demographics, details from GP visits, diagnoses and comments from specialists’ referrals and hospital admissions, prescriptions, laboratory tests and additional information including height, weight and smoking status. The Read classification is used to code diagnoses,20 and a drug dictionary based on the Multilex classification is used to code drugs.21 GPs receive specific training and regular feedback for accurate data recording;22 quality of THIN data has been shown to be high19 and valid for use in pharmacoepidemiological research.23 This study was approved by the UK National Health Service Multicentre Research Ethics Committee.
Study design
We conducted two cohort analyses: (1) incident MIs and strokes, and (2) incident PVD, among individuals with SSc (SSc cohorts) as compared with age-, sex- and entry time-matched individuals without SSc (comparison cohorts) using data from THIN. These analyses were conducted separately because the aetiological factors that lead to MIs and strokes may differ from those that lead to PVD, particularly in SSc, where previous studies have suggested that the prevalence in SSc patients may be increased for the latter but not for the former.24 ,25
For each individual with SSc, we matched up to 10 patients who have not had an SSc diagnosis at any time, based on age, sex and month/year of cohort entry. Our study spanned the period of 1 January 1986 through 30 September 2011. Follow-up began after the first diagnosis of SSc for the SSc cohorts, and the matched date (index date) in the comparison cohorts. For both SSc and comparison cohorts, age greater than 18 years and continuous enrolment in the database for at least 12 months prior to start of follow-up was required.26 Individuals who had a record documenting a history of or occurrence of coronary artery or cerebrovascular events (MIs, unstable angina, strokes and coronary or carotid revascularisation procedures) prior to the start of follow-up were excluded from the outcome analyses of MIs and strokes. In the same manner, individuals who had PVD prior to the start of follow-up were excluded for the analysis of the PVD outcome. Patients were followed until they experienced the outcomes of interest, died, left the THIN database or the follow-up ended (30 September 2011), whichever came first.
Definition of SSc
For inclusion into the SSc cohorts, a list of diagnosis records was used to identify all patients with a diagnosis of SSc. These included ‘scleroderma’, ‘systemic sclerosis’, ‘progressive systemic sclerosis’, ‘CREST’, ‘myopathy due to scleroderma’ and ‘lung disease due to systemic sclerosis’. Individuals identified by a record for ‘scleroderma’ were excluded if they also had a record for localised scleroderma (morphoea) (N=44). We performed a secondary analysis by excluding individuals in the SSc and comparison cohorts who also had a diagnosis record of SLE or RA on or after the index date (indicating possibly overlap features in the SSc cohorts), since SLE and RA are known to increase the risk of CVD.
The method of using codes (ICD-9) to identify SSc patients from a database has been successfully employed in another population-based US study, yielding an incidence rate of SSc that was similar to other validated US estimates in the literature.27 Further support for the accuracy of SSc coding comes from a validation study of ICD-9 coding in an administrative database, where the specificity of SSc diagnosis was high, at 94.9% (95% CI 93.0 to 96.2).28 Physicians’ diagnosis records of SSc in THIN, as opposed to billing codes, are expected to be even more accurate since they can be made after sufficient information (ie, lab results and consultations) has been collected, and corrected if necessary. SSc, in particular, being a disease with such distinct physical features and potentially grave prognostic implications for the patient, is expected to be accurately recorded.
Ascertainment of outcomes
An individual was considered to have had an MI or stroke at the first recording of any Read term synonymous with these diagnoses.29–32 Diagnosis records that specified angina, transient ischaemic attacks, haemorrhagic strokes,33 or sudden deaths were not included. PVD was defined as the first recording of PVD or claudication; arterial thromboembolism, occlusion, bypass or angioplasty of the lower extremities; and ischaemia or amputation of the legs or feet.33 ,34 Diagnosis records that specified arterial disease of the upper extremities or toes were not included to minimise inadvertent inclusion of the consequences of Raynaud's phenomenon.
Assessment of covariates
Cardiovascular risk factors were assessed prior to the start of follow-up based on the presence of relevant codes. Medication use was defined by the presence of a prescription in the 12 months prior to the start of follow-up. Diabetes and hyperlipidaemia were defined by the presence of a diagnosis record or a prescription of medications specific for the treatment of these respective diseases.35 Cardiovascular risk factors have been successfully used in previous analyses in THIN by confirming anticipated associations such as those among smoking and lung cancer;36 and body mass index (BMI), diabetes, hypertension, hyperlipidaemia and MI.23
Statistical analyses
We compared the baseline characteristics across the SSc and comparison cohorts. In the cohort for MI and stroke, we identified cases of MIs and strokes during follow-up and calculated person-years (PY) to estimate incidence rates (IRs), both individually and as a composite outcome (ie, MI or stroke). We performed the same analysis in the cohort for PVD events and estimated IRs for PVD. We estimated the cumulative incidence of each event accounting for the competing risk of death37 by using the SAS macro CUMINCID and graphically presented these results. We employed Cox proportional hazard regression models to assess multivariable HRs after stratifying by matched variables (age, sex and time of cohort entry). We imputed missing values for BMI and smoking using IVEware for SAS, V.9.2 (SAS Institute, Cary, North Carolina, USA).38 We imputed five datasets and then combined estimates from these datasets.39 Our multivariable analyses were adjusted for BMI (WHO categories, in kg/m2), smoking (never, past, and current), hypertension, diabetes, hyperlipidaemia and use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) and oral glucocorticoids. Atrial fibrillation was adjusted for in the analysis of stroke and PVD because it is a risk factor for these outcomes.40 ,41 For all HRs, we calculated 95% CI. All p values were two-sided.
Results
Myocardial infarction and stroke
From 39 782 608 PY of follow-up in the THIN, 915 individuals with a first recorded diagnosis of SSc were identified. After exclusion of individuals with prior coronary artery and cerebrovascular events, 865 individuals with SSc (85.8% women, mean age 58.7 years) were included in the cohort analysis for MI and stroke. The baseline characteristics of the study cohorts for this analysis are shown in table 1. The SSc cohort had lower frequencies of overweight and obese status, more frequent past smoking status, and more frequent use of aspirin, NSAIDs and oral glucocorticoids.
Characteristics of systemic sclerosis (SSc) and comparison cohort at baseline†
The mean follow-up time was 5.2 years in the SSc cohort and 6.0 years in the comparison cohort. In the SSc cohort, new diagnoses of MI and stroke occurred in 20 and 22 individuals over 4557 and 4555 PY of follow-up, respectively (table 2). SSc was associated with an increased risk of MI and stroke. Relative to the comparison cohorts, the age-, sex- and entry time-matched HRs were 1.97 (95% CI 1.21 to 3.22) for MI and 2.56 (95% CI 1.58 to 4.14) for stroke. After adjusting for BMI, smoking, hypertension, diabetes, hyperlipidaemia, aspirin use and atrial fibrillation (for the stroke outcome only), the HR for MI was attenuated slightly, but not for stroke. After further adjustment for NSAID and oral glucocorticoid use, the HRs were 1.80 (95% CI 1.07 to 3.05) for MI and 2.61 (95% CI 1.54 to 4.44) for stroke. When we excluded individuals with SLE or RA diagnoses on or after the index date, the final adjusted HR was 1.93 (95% CI 1.12 to 3.33) for MI and 2.57 (95% CI 1.50 to 4.42) for stroke (see online supplementary table S1).
HRs of incident myocardial infarction (MI) and stroke according to systemic sclerosis (SSc) status
Peripheral vascular disease
In our analysis for PVD, 858 individuals with SSc (85.3% women, mean age 58.9 years) were included after exclusion of individuals with prior PVD. The baseline characteristics of the SSc and comparison cohorts were similar to those for the MI and stroke analysis (see online supplementary table S2). The mean follow-up time was 5.2 years in the SSc cohort and 5.9 years in the comparison cohort. In the SSc cohort, a new diagnosis of PVD occurred in 34 individuals over 4462 PY of follow-up (table 3). Relative to the comparison cohort, the age-, sex- and entry time-matched HR for PVD was 4.57 (95% CI 2.99 to 7.01). After adjusting for BMI, smoking, hypertension, diabetes, hyperlipidaemia, aspirin use and atrial fibrillation, this HR remained similar. After further adjustment for NSAID and oral glucocorticoid use, the HR was slightly reduced to 4.35 (95% CI 2.74 to 6.93). When we excluded individuals with SLE or RA diagnoses on or after the index date, the final adjusted HR was 4.88 (95% CI 3.00 to 7.94) (see online supplementary table S3).
HRs of incident peripheral vascular disease according to systemic sclerosis (SSc) status
Discussion
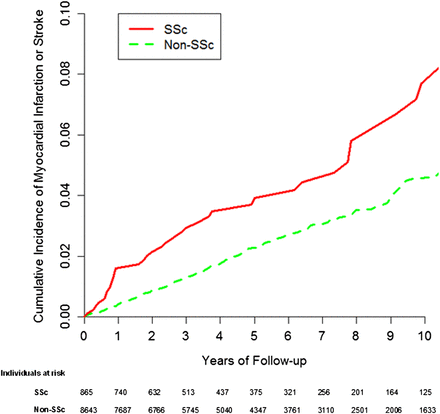
In this population-based cohort of patients with SSc, the risks of incident MI and stroke were increased approximately two-fold compared with those without SSc (figure 1), with the risk of stroke being greater than that of MI. Individuals with SSc also had a four-fold increased risk of PVD (figure 2). These associations persisted after adjustment for known and suspected cardiovascular risk factors, and also did not change materially after excluding individuals with possibly SLE or RA overlap. These findings provide the first general population-based evidence that SSc is associated with an increased risk of developing MI, stroke, and PVD, and shed light on corresponding risk magnitudes associated with these CVD outcomes.
Cumulative incidence of myocardial infarction or stroke in 865 individuals with systemic sclerosis (SSc) as compared with 8643 age-, sex-, entry time-matched, non-SSc individuals. Estimates accounted for the competing risk of death. This figure is only reproduced in colour in the online version. This figure is only reproduced in colour in the online version.
Cumulative incidence of peripheral vascular disease in 858 individuals with systemic sclerosis (SSc) as compared with 8580 age-, sex-, entry time-matched, non-SSc individuals. Estimates accounted for the competing risk of death. This figure is only reproduced in colour in the online version. This figure is only reproduced in colour in the online version.
Many studies have examined for subclinical radiographic evidence of atherosclerosis (ie, carotid IMT, brachial artery flow mediated vasodilation, intracerebral and coronary calcification on CT) in SSc. A systematic review of such studies concluded that the prevalence of atherosclerosis was increased in all vessels studied in individuals with SSc.9 The data were most robust for carotid atherosclerosis identified by ultrasound, which has been shown in the general population to confer an increased risk of coronary artery disease, strokes and death.42 The results of our study expand upon these findings of subclinical atherosclerosis and provide support that the risk of clinically relevant cardiovascular events in SSc is increased as well.
Our findings are in general agreement with studies that have examined prevalences of CVD in individuals with and without SSc. For example, a cross-sectional study of 31 patients with limited cutaneous SSc versus 31 controls reported that the prevalences of coronary artery and cerebrovascular disease were increased 1.7 and 1.3 times, respectively.24 However, these estimates were not statistically significant, likely due to the study's small sample size. Recently, another cross-sectional study compared the prevalences of coronary artery disease in the Australian Scleroderma Cohort Study with two external population-based cohorts, and found that SSc patients had over three times the prevalence of coronary artery disease.13 However, this study did not address stroke or PVD and the authors noted potential selection of patients with greater disease severity in their SSc cohort given their referral-based cohort recruitment.13
Several studies have also reported that PVD is more prevalent in SSc patients. Youssef and colleagues showed that PVD was six times as prevalent in SSc patients versus controls based on a chart review.24 Our estimate is similar to another study that assessed intermittent claudication using a WHO questionnaire and found that the prevalence in SSc patients was 4.7 times as high compared with that of an external comparison cohort.43 The same investigators subsequently performed a study using an ankle brachial pressure index (ABPI) cut-off of <0.944 to define PVD, showing that 17% of SSc patients had PVD compared with none in controls.45
The increased risk of cardiovascular events found in our study may be due to atherosclerosis. Alternatively, these events may represent the effects of vasospasm, SSc specific vasculopathy, vasculitis, thrombosis or a combination of atherosclerotic and non-atherosclerotic factors. Furthermore, the mechanisms that lead to MI, stroke or PVD may be different in SSc, which may explain the higher observed risk for PVD, and slightly higher risk of stroke compared with MI. There are multiple possibilities as to why patients with SSc may develop increased atherosclerosis. Endothelial dysfunction, which is central to both SSc pathophysiology and atherogenesis,5 ,46 is associated with oxidative stress, resulting in reactive oxygen species (ROS) production.8 ROS oxidises low-density lipoprotein (LDL), and the subsequent consumption of oxidised LDL (ox-LDL) by monocytes is what leads to the formation of atherogenic foam cells.3 Although foam cells have not been studied in SSc, circulating ox-LDL/β2-glycoprotein 1 complexes47 and anti-ox-LDL antibodies may be elevated in SSc patients.48 SSc patients may also have higher levels of atherogenic proinflammatory high-density lipoprotein (piHDL) and lipoprotein(a).12 ,49
By contrast, the prevalence of traditional CVD risk factors does not seem to be increased in SSc.13 Blood pressure has been shown to be similar between SSc and controls.50 The frequencies of obesity, hyperlipidaemia, hypertension, and diabetes were not increased in SSc relative to comparison cohorts in our study, which is similar to what other studies of atherosclerotic disease in SSc patients have found.7 ,11 ,13 Still, other disease-specific factors such as medication use and lack of exercise may be contributing factors to atherosclerotic disease. In this study, the HRs of MI and PVD were slightly decreased when NSAID and oral glucocorticoid use were adjusted for, indicating that either these medications were contributing to CVD risk, or that the indications for these medications (eg, prominent inflammatory features of SSc) at baseline were associated with CVD risk. The additional hemodynamic stresses conferred by pulmonary hypertension13 and interstitial lung disease, or potential stroke risk due to SSc related arrhythmias may also play a role.
The strengths of our study include the use of a large database that provided sufficient power to study a rare disease, SSc, in a general population context. At the same time, we had access to information on cardiovascular risk factors and outcomes over many years. Several limitations deserve comment. Uncertainty surrounding diagnostic accuracy is a potential concern in studies that identify cases from databases. However, THIN data, as opposed to administrative records, consist of medical records that physicians use for patient care, and thus the overall accuracy is expected to be higher, as reflected in many validation studies of important outcomes.32 ,51 ,52 Misclassification of SSc in particular is expected to be rare given the distinct physical features of the disease, and the specificity of SSc diagnosis even in an administrative database has previously been shown to be high, at 94.9%.28 In addition, the incidence rate of SSc found in our study of 23 per million PY is similar to several other estimates in the literature.17 ,53 ,54 While MIs and strokes are expected to be coded with high accuracy in THIN,31 ,32 misclassification of PVD could occur if physicians mislabel a patient's Raynaud's symptoms as PVD despite our attempt to minimise this possibility. Nevertheless, it is reassuring that the risk estimates of PVD in SSc obtained from other studies using ABPI and questionnaires as discussed above were either higher than,45 or similar,43 respectively, to ours. We did not have access to disease specific information such as SSc subtype or organ involvement, which limited our ability to correlate such characteristics with the studied outcomes. As our study focused on the main links between SSc and the risk of CVD, these findings call for future studies aimed at identifying the risk factors for CVD, including disease specific features or medications among individuals with SSc. Furthermore, it would be valuable to examine extended aspects of CVD outcomes including their severity and consequences.
In conclusion, this general population-based cohort study suggests that SSc is associated with an increased risk of MI, stroke, and PVD. Our findings support the consideration of CVD disease prevention and surveillance in SSc patients, as CVD risk modification represents an opportunity to further reduce the morbidity and mortality of SSc.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
-
Contributors All authors participated in the conception, design and analyses of the study. AM and HKC drafted the manuscript and are guarantors. All authors contributed to interpretation of the results.
-
Funding This work was supported in part by grants from the NIAMS (P60AR047785) and Boston University School of Medicine. AM is a recipient of the Roche Canada Scleroderma and Vasculitis Fellowship and the Gurmej Kaur Dhanda Scholarship award. The funding sources had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
-
Competing interests None.
-
Ethics approval This study was judged exempt by the Institutional Review Board at Boston Medical Centre, and was approved by the National Health Service Multicentre Research Ethics Committee.
-
Provenance and peer review Not commissioned; externally peer reviewed.