Article Text
Abstract
Objectives To explore the role of individual and country level socioeconomic (SE) factors on employment, absenteeism and presenteeism in patients with spondyloarthritis (SpA) across 22 countries worldwide.
Methods Patients with a clinical diagnosis of SpA fulfilling the ASAS classification criteria and in working age (≤65 years) from COMOSPA were included. Outcomes of interest were employment status, absenteeism and presenteeism, assessed by the Work Productivity and Activity Impairment Specific General Health questionnaire. Three multivariable models were built (one per outcome) using mixed-effects binomial (for work status) or ordinal regressions (for absenteeism and presenteeism), with country as random effect. The contribution of SE factors at the individual-level (eg, gender, education, marital status) and country-level (healthcare expenditure (HCE) per capita, Human Development Index (HDI) and gross domestic product per capita) SE factors, independent of clinical factors, was assessed.
Results In total, 3114 patients with SpA were included of which 1943 (62%) were employed. Physical function and comorbidities were related to all work outcomes in expected directions and disease activity also with absenteeism and presenteeism. Higher education (OR 4.2 (95% CI 3.1 to 5.6)) or living in a country with higher HCE (OR 2.3 (1.5 to 3.6)) or HDI (OR 1.9 (1.2 to 3.3)) was positively associated with being employed. Higher disease activity was associated with higher odds for absenteeism (OR 1.5 (1.3 to 1.7)) and presenteeism (OR 2.1 (1.8 to 2.4)). No significant association between individual-level and country-level SE factors and absenteeism or presenteeism was found.
Conclusions Higher education level and higher country SE welfare are associated with a higher likelihood of keeping patients with SpA employed. Absenteeism and presenteeism are only associated with clinical but not with individual-level or country-level SE factors.
- spondyloarthritis
- economic evaluations
- epidemiology
- outcomes research
Statistics from Altmetric.com
Introduction
With a worldwide ageing population, the prevalence and consequences of chronic and debilitating diseases have been increasing.1 Countries are challenged to invest in health and healthcare to keep their citizens active, in order to increase productivity and prolong the lifetime of efficient work. In turn, this strategy has been shown to generate wealth that can be used to improve the welfare system generating a ‘positive cycle’. These societal aspirations make no exception for patients with chronic disease such as spondyloarthritis (SpA).2
Research on the economic impact of SpA has consistently shown that indirect costs due to sick leave and work disability are, by far, the major sources of the total SpA-related cost of illness.3–7 Biologic drugs were able to improve absenteeism (sick leave), presenteeism (reduction on performance while at work because of health reasons)8 and employment status.9 However, despite optimal drug treatment, persons with SpA still experience restrictions in work participation, and a role for personal and environmental contextual factors has been suggested.10 Also, while most studies on work outcomes have been performed in Western societies, the necessities in relation to work outcome are likely different across countries.
On this line, there is a need to better understand the variation of the different work outcomes across countries and to gain insight into the role of personal as well as country-level contextual factors, and specifically of socioeconomic (SE) characteristics.11 12 This knowledge can be used to plan and implement better health policies for patients with SpA, and may improve work participation and reduce the economic burden. Cohorts including patients from several countries, such as the Assessment in SpondyloArthritis international Society (ASAS) Evaluation of co-morbidities in spondyloarthritis (COMOSPA) study with patients from 22 countries from all world regions, are scarce but essential to understand these effects.13 Two studies in SpA that compared work outcome between countries suggested large differences.3 6 14 However, as only two or three countries were compared, it was not possible to explore systematic effect related to country of residence.
Our aims were twofold: first, to assess the impact of clinical and individual SE characteristics on employment status, absenteeism and presenteeism12 among patients with SpA at working age, while accounting for country of residence; second, to explore the potential role of specific country-level SE factors on these outcomes.
Methods
Patients and study design
The ASAS-COMOSPA study design and procedures have been previously described.13 Briefly, this was a large, cross-sectional, multicentre, international study, with 22 participating countries from four continents (Africa, America, Asia and Europe). Consecutive adult patients (≥18 years old) with a clinical diagnosis of SpA according to their rheumatologist,15 16 and able to understand and complete questionnaires were included. For the current study, only patients of working age (≤65 years old) were included.
The study was conducted according to guidelines for good clinical practice in all countries. Written informed consent was obtained from all subjects before enrolment.
Data collection
Outcomes
Work participation, more specifically employment, absenteeism and presenteeism, were assessed according to the Work Productivity and Activity Impairment questionnaire General Health (WPAI-GH).17
The WPAI-GH has six questions, the first asking about the employment status (binary; yes/no). The subsequent five questions allow to calculate for the past 7 days, the percentage of time absence from the workplace (absenteeism, 0%–100%), the percentage of loss of productivity while at work (presenteeism, 0%–100%) and the percentage of overall work impairment (0%–100%) in those employed, as well as the percentage of activity impairment (0%–100%) in all patients.
For the present study, only the employment status, absenteeism and presenteeism were assessed. Because of a skewed distribution (and zero inflated for absenteeism), the last two outcomes were categorised into three (0%; >0%–20%; >20%–100%) and four categories (0%; >0%–20%; >20%–50%; >50%–100%), respectively. The number of categories and cut-points was based on preliminary analysis.
Demographic, clinical characteristics and individual SE factors
A standardised case report form was used by a local researcher or by the rheumatologist to collect the following variables: (1) individual SE factors: age, gender, education (as a categorical variable: primary education, secondary and university) and current marital status (as a categorical variable: single, married or living together, divorced and widow); (2) lifestyle: body mass index, smoking status (past and current); (3) SpA characteristics: disease duration, peripheral arthritis, enthesitis, dactylitis, human leucocyte antigen B27 (HLA-B27), SpA phenotype (axial (imaging vs clinical arm) vs peripheral) and extra-articular manifestations (inflammatory bowel disease, uveitis and psoriasis); (4) SpA activity and severity measures: Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Ankylosing Spondylitis Disease Activity Score (ASDAS) calculated with C reactive protein, number of swollen and tender joints (44 joint count), the presence of ‘bamboo spine’ and the Bath Ankylosing Spondylitis Functional Index (BASFI); (5) comorbidities: assessed by the Rheumatic Diseases Comorbidity Index (RDCI)18 19; (6) past and current medications: non-steroidal anti-inflammatory drugs, oral steroids, conventional synthetic and biological disease-modifying antirheumatic drugs; and (7) imaging: sacroiliitis on pelvic radiographs assessed according to the modified New York (mNY) criteria20 and sacroiliitis on MRI defined based on the ASAS criteria,21 both according to the treating rheumatologist.
RDCI was completed with data from the CRF with physician-reported data about stroke and patient-reported data about heart diseases, hypertension, lung diseases, stomach ulcers, cancer and fractures.
Country-level SE characteristics
The following country-level SE characteristics were obtained: (1) healthcare expenditure (HCE) per capita (source: World Bank 2013) in International dollars (Intl$) to adjust for purchasing power parities (HCE-PPP); (2) Human Development Index (HDI) (source: United Nations Development Program 2013); (3) GDP per capita (source: International Monetary Fund 2013) in int$ (GDP-PPP); (4) unemployment rate (%) of the total labour force (source: World Bank 2013; estimate from International Labour Organization).22–26 Detailed information on SE data per country is provided in online supplementary table S1.
Supplemental material
Statistical analyses
For analysis of work status, the total sample of patients with SpA of working age (≤65 years old) were included, while for the analysis of absenteeism and presenteeism, the subsample of employed subjects were considered. Patients reporting 100% absenteeism were excluded from the analysis of presenteeism.
Country-level SE variables were divided into tertiles, and the lowest tertiles (reference level) were compared with the medium and the highest tertiles combined.
Possible associations between all demographic, clinical factors (lifestyle, SpA characteristics, SpA activity and severity, and comorbidities), as well as individual-level SE factors, and the three work outcomes were first explored in univariable models. Variables with a p value <0.20 were selected for the multivariable models. For employment status, binomial mixed-effects regression with country as random effect (RE) was applied and ordinal mixed-effects models, also with country as RE, were used for absenteeism and presenteeism. The final models included variables that were significantly associated with the outcome of interest (p<0.05).
Each country-level SE variable (HCE-PPP, HDI, GDP-PPP, unemployment rate) was added, in separate models (because of collinearity), to the three multivariable models to assess their possible independent effect on work outcomes (significant if p<0.05), additional to the effect of demographic, clinical and individual-level SE factors.
The inferential analysis was performed on complete cases.
All analyses were performed using Stata V.14.
Results
Patient characteristics
In total, 3114 patients from 22 countries (Argentina, Belgium, Canada, China, Colombia, Egypt, France, Germany, Italy, Japan, Mexico, Morocco, Netherlands, Portugal, Russia, Singapore, South Korea, Spain, Taiwan, Turkey, UK and USA) were included.
Demographic, clinical and imaging characteristics of the study population, as well as the comparison between employed and unemployed patients, are shown in table 1. In this study, 1943 patients (62%) were employed. Of note, a large proportion of patients reported a university-level of education, which was higher among employed (50%) as compared with unemployed patients (35%).
Patient and disease characteristics according to employment status
One quarter (n=508, 27%) of the employed patients had been absent from their workplaces more than 20% of the time during the previous 7 days (absenteeism), but almost half (n=803, 47%) of those who were present felt that their disease reduced their productivity (presenteeism) by more than 20% (table 1).
All work outcomes varied across countries and are presented in table 2. Employment was lowest in Colombia (28.1%) and highest in Canada (83.3%), absenteeism was lowest in South Korea (1.2%) and highest in Germany (53.1%), and presenteeism, impact on productivity, was lowest in Japan (17.8%) and highest in Germany (42.3%).
Work outcomes and therapy per country
Effect individual SE factors on work outcomes
The effect of individual SE and clinical characteristics on each of the three outcomes in the final multilevel models is shown in table 3. Patients with a higher level of education were more likely to be employed (model 1: OR 4.2 (95% CI 3.2 to 5.6)). This protective effect was not observed either for absenteeism (model 2) or presenteeism (model 3). In addition, male gender, being married or living together or being divorced (compared with being single), a better function and a lower number of comorbidities were associated with a higher likelihood of being employed (model 1) but not with absenteeism or presenteeism (model 2 and model 3).
Sociodemographic, clinical and individual socioeconomic factors associated with work outcomes*
More comorbidities and a worse function (BASFI) were associated with lower employment (model 1) and a higher odds for absenteeism (model 2) and presenteeism (model 3). In addition, higher disease activity was associated with higher odds for absenteeism (model 2) and presenteeism (model 3).
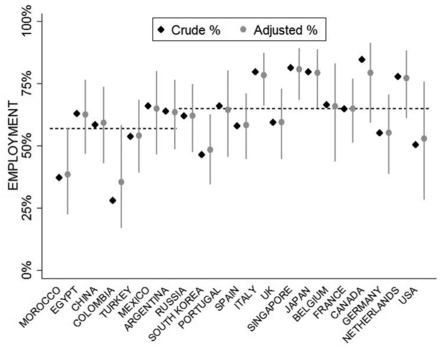
The distribution of employment across countries (unadjusted and adjusted (model 1)) is described in figure 1.
Percentage of employment by country (n=21; no data available on healthcare expenditure for Taiwan). Crude %: observed employment per country; adjusted %: estimated from model 1 (table 3). Countries ordered by healthcare expenditure per capita (from lowest to highest). The dashed horizontal lines represent the predicted % of employment for countries with a low healthcare expenditure (<1500 Intl$ per year per capita) and with a high health expenditure (≥1500 Intl$ per year per capita).
Of note, there was no significant association between classification status (axial SpA (axSpA) vs peripheral SpA (pSpA)) and any of the studied outcomes (univariable p values range: 0.19–0.99), or between the ‘arms’ of the axSpA criteria (imaging arm±clinical arm vs clinical arm alone) and these outcomes (univariable p values range: 0.22–0.81).
Discussion
In this large international study, we have first shown the importance of individual SE factors (ie, education and gender), in addition to clinical characteristics on work participation among patients with SpA. Notwithstanding, these ‘individual factors’ fail to fully explain the large variability of work outcomes across countries. Our results emphasise variation in work outcomes in patients with SpA across countries and suggest that country-level SE factors also play an important role, especially the countries’ wealth and the level of investment in the healthcare system.
In all final multivariable models, the random effect of country on work outcomes was still significantly greater than zero. This indicates that, after adjusting for the clinical and individual SE factors included, there are likely to be further unmeasured variables that influence work outcomes. These may be country-dependent variables that we were unable to include, such as availability and generosity of sickness benefit, or individual-level variables that vary across countries, such as the type of work and work-related stress.
A recent systematic literature review from the Outcome Measures in Rheumatology (OMERACT) summarised the evidence on the relationship between individual-level and country-level SE factors on work status, absenteeism and presenteeism in patients with radiographic axSpA (r-axSpA).10 Between-study differences rendered comparisons challenging. Overall, while evidence about the effect of gender and education on work status was conflicting, there was insufficient evidence about their role on presenteeism and absenteeism, and no evidence on the role of country of residence on any work outcome.7 27–29
Our study revealed that the individual SE factors are associated with employment status, but not with absenteeism and presenteeism. With regard to gender, this might not be surprising, as it likely reflects that in many countries population employment among women with an average age of 40 (SD 12) years is still lower compared with men. Of note, among those employed, gender has no influence on absenteeism or presenteeism.30 Similarly, a positive relation between higher education or marital status was only seen for employment, but not for absenteeism and presenteeism. Our data suggest that the influence of disease activity and severity on these latter outcomes overshadows the role of personal contextual factors.
The aforementioned review emphasised the lack of data to quantify and understand country differences in work outcomes. Notwithstanding, this would be of importance for, among others, planning national initiatives to improve work participation in patients with chronic disease or European-level policies. Results from the Outcomes Assessment in AS International Study (OASIS) in patients with r-axSpA across three European countries (Belgium, France and The Netherlands) has shown differences between countries on employment and presenteeism, adjusting for individual sociodemographic and clinical characteristics.4 29 31 Country of residence included as a categorical variable revealed to be associated with the outcomes.4 However, the study only concerned three European countries, and the role of country-level SE characteristics was not explored when trying to explain the effect of country of residence. A study by Mau et al revealed that employment in the former Eastern German states was lower than in former Western German states. The higher standardised employment among patients with ankylosing spondylitis was attributed to a higher unemployment in former Eastern Germany. However, this was not formally tested in the analyses.14
In the current study, we have found large variations in all work outcomes across countries and showed that countries’ welfare (especially HCE or HDI) is associated with a higher likelihood of being employed among patients with SpA. Associations between country-level SE status and absenteeism or presenteeism were less clear.
Wealthier and more developed countries apparently invest more in health and also in healthier workplaces, in efforts to support (chronically ill) persons to remain healthy during their working careers. Notwithstanding, even in wealthier countries, employed patients cannot avoid taking sick leave or experiencing productivity loss in case of higher disease activity.
It should be noted that the determinant factor for reaching statistical significance may be the number of included countries, which, though impressive (n=22), statistically speaking is not so high and may therefore lead to power issues when analysing country-level variables. In other words, the effects found, namely the positive association between higher HCE or higher HDI and higher likelihood of employment, may be an underestimation of the real effect of country-level SE factors on work outcomes in SpA. Similar results have already been seen in rheumatoid arthritis (RA). The Comorbidities in Rheumatoid Arthritis (COMORA) international study assessed the same work outcomes as in COMOSPA but in patients with RA across 17 countries. Similar to our results, this study has also shown that there are substantial differences in work outcomes among patients from different countries, and that this difference is independent of clinical and individual SE factors.32 Patients with RA, living in a country with a lower economic wealth and a lower human development of countries had a higher chance to have no employment, higher absenteeism, but paradoxically lower presenteeism. Our results were identical for employment. However, no association could be found for the other two outcomes.
To the best of our knowledge, this is the first study to assess the effect of both individual-level and country-level SE factors in a worldwide setting and in patients within the full spectrum of SpA. Of note, there was no association between the ‘SpA phenotype’ (axSpA vs pSpA) and work-related outcomes, as previously found in a smaller Swedish study.33 In addition, being mNY-positive had no effect on work participation and we also found no measurable differences in work outcomes between those fulfilling the clinical and imaging ‘arms’ of the ASAS axSpA classification criteria. It has been consistently shown that patients with r-axSpA and nr-axSpA have similar disease burden34 and respond similarly to therapy.35 In addition, the ‘clinical arm’ of the axSpA criteria has been shown to belong to the ‘SpA Gestalt’ as much as the imaging ‘arm’ contrary to what has been initially claimed.36 37 Our results yield further evidence in favour of the full ‘SpA spectrum’, by showing, for the first time, similar work outcomes across the different ‘sub-groups’ of the disease spectrum.
Consistent with other studies, the association between BASFI and comorbidities with work outcomes is confirmed.7 27 29 33 Since fewer studies are available on presenteeism and absenteeism, it is important to highlight the additional, and expected, strong role of ASDAS in sick leave and presenteeism during the last 7 days.
Our study has some limitations worth noticing. First, being a cross-sectional study the direction of the associations found cannot be determined, especially for comorbidities and for the role of disease activity on employment. However, this is the largest yet worldwide study performed to determine factors associated with work outcomes in the full spectrum of SpA and provides relevant data to inform future longitudinal studies. Second, some countries may be under-represented due to their small sample size (eg, Belgium, Canada and Colombia). Thus, caution should be taken when extrapolating our results to specific countries. However, all available data could efficiently be taken into account in our analysis using sophisticated multilevel analytical methods. With this method, there is no need for between-countries stratification and the resulting loss of statistical power. Third, although we have used data from the well-known COMOSPA study, information bias cannot be completely ruled out. In fact, we found a larger-than-expected number of patients with university-level education (1375 or 44%). Fourth, even though consecutive patients were included and the baseline characteristics are representative of a SpA population, selection bias cannot be excluded, and the patients may therefor not fully reflect the real SpA population. Fifth, there are no work-related variables that are likely to be important predictors of work participation
In conclusion, we have shown, using international data, the relevance of the much-overlooked individual-level and country-level SE factors on work participation in patients with SpA. Investing in the healthcare system leads to better work outcomes irrespective of the patient’s individual characteristics including disease activity and therapy. Although longitudinal data are warranted, our results suggest that health policies taking both individual-level and country-level SE factors into account may, more effectively, promote work participation among patients with SpA.
Effect of country-level socioeconomic factors on work outcomes*
Acknowledgments
This study was conducted under the umbrella of the International Society for Spondyloarthritis Assessment (ASAS).
References
Footnotes
Handling editor Josef S Smolen
Contributors SARM wrote the first draft of the manuscript, which was then reviewed and edited by AS, SR, FPS, PP, EN, SN, AM, MD, DvdH, RBML, FEvdB and AB. The study was conceptualised by SARM, AS, SR, FPS, PP, EN, SN and AB. The statistical analyses were carried out by SARM with the advice and support from AS, SR, FPS, PP, EN, SN and AB.
Funding The COMOSPA study was conducted with the financial support of Abbvie, Pfizer and UCB, who provided an unrestricted grant to ASAS to fund the study.
Disclaimer The funders did not have any role in the design or conduct of the study. This ancillary study did not receive any funding, and the sponsors of COMOSPA did not have any interference with this current study.
Competing interests None declared.
Patient consent Not required.
Ethics approval The study was conducted according to guidelines for good clinical practice in all countries with all local ethics committees approving the ASAS-COMOSPA study protocol.
Provenance and peer review Not commissioned; externally peer reviewed.
Collaborators (1) Fadoua Allali, MD, Mohamed Vth University, URAC 30, Department of Rheumatology, El Ayachi Hospital, Salé, Faculty of Medicine and Pharmacy, Rabat, Morocco. fadouaallali@yahoo.fr (2) Raquel Almodovar González, MD, Hospital Fundación Alcorcón, Madrid, Spain. ralmodovar@fhalcorcon.es (3) Elena Alonso Blanco-Morales, MD, Hospital Universitario Juan Canalejo, La Coruña, Spain. elena.alonso.blanco.morales@sergas.es (4) Alejandro Alvarellos, MD. aalvarellos@gmail.com Hospital Privado de Córdoba, Argentina. (5) Maria Aparicio Espinar, MD, Hospital Universitario Bellvitge, Barcelona, Spain. maparicioe@bellvitgehospital.cat (6) Pamir Atagunduz, MD, Division of Rheumatology, Marmara University, Faculty of Medicine, Istanbul, Turkey. (7) Pauline Bakker, MD, Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands. P.A.C.Bakker@lumc.nl (8) Juan C. Barreira, MD, jbarreira@hbritanico.com.ar, Hospital Británico de Buenos Aires, Argentina. (9) Leila Benbrahim, MD, Mohamed Vth University, URAC 30, Department of Rheumatology, El Ayachi Hospital, Salé, Faculty of Medicine and Pharmacy, Rabat, Morocco. l.benbrahim@yahoo.fr (10) Bahia Benchekroun, MD, Mohamed Vth University, URAC 30, Department of Rheumatology, El Ayachi Hospital, Salé, Faculty of Medicine and Pharmacy, Rabat, Morocco. benchk_1@yahoo.fr (11) Alberto Berman, MD. Albertoberman1@yahoo.com.ar, Centro Médico Privado, Tucumán, Argentina. (12) Juergen Braun, MD, Rheumazentrum Ruhrgebiet, Herne, Germany. juergen.braun@elisabethgruppe.de (13) Alain Cantagrel, MD, PhD, Centre de Rhumatologie, CHU Purpan, Toulouse, France. (14) Roberto Caporali, MD, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy. (15) Pedro Carvalho, MD, Clínica Universitária de Reumatologia, Hospitais da Universidade de Coimbra, Coimbra, Portugal. (16) Gustavo Casado, MD. guscasado@hotmail.com. Hospital Militar Central, Buenos Aires, Argentina. (17) James Cheng-Chung Wei, MD, PhD, Chung Shan Medical University Hospital, Taichung, Taiwan. e-mail: jccwei@gmail.com (18) Francisco Colombres, MD. colombresfrancisco@yahoo.com.ar. Centro Médico Privado, Tucumán, Argentina. (19) Eugenio del Miguel Mendieta, MD, PhD, Hospital Universitario La Paz, Madrid, Spain. eugenio.demiguel@gmail.com (20) Juan D. Diaz-Garcia, MD, Escuela Superior de Medicina, Instituto Politécnico Nacional, Mexico. (21) Michel De Bandt, MD, PhD, Rheumatology Unit, University Hospital of Martinique, Fort de France, France. (22) Vanesa Duarte, MD. vanu.duarte24@gmail.com. Hospital Rivadavia, Buenos Aires, Argentina. (23) Cristina Fernandez Carballido, MD, Hospital General Universitario de Elda, Elda, Spain. soficarballido@hotmail.com (24) Mari Cruz Fernandez Espartero, MD, Hospital de Mostoles, Madrid, Spain. mcruzespartero@yahoo.es (25) Manuel Fernandez-Prada, MD, Hospital de Guadalajara, Guadalajara, Spain. (26) Rene-Marc Flipo, MD, PhD, Rheumatology Department, CHRU Lille, France. (27) Pilar Font Ugalde, MD, PhD, Hospital Universitario Reina Sofia, IMIBIC, Córdoba, Spain. fougp@hotmail.com (28) Philippe Gaudin, MD, PhD, Clinique Universitaire de Rhumatologie, Hôpital Sud, Echirolles, and Université Joseph Fournier, Grenoble, France. (29) Philippe Goupille, MD, Rheumatology Department, Tours University, Tours, France. (30) Dolors Grados Cánovas, MD, Hospital San Rafael, Barcelona, Spain. dgrados23@hotmail.com (31) Jordi Gratacós Masmitjá, MD, PhD. Hospital Universitario Parc Taulí, Badalona, Spain. jgratacosmas@gmail.com (32) Vittorio Grosso, MD, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy. (33) Naomi Ichikawa, MD, Institute of Hematology, Tokyo Women’s Medical University, Japan. Naomu@twmu.ac.jp 34. Hisashi Inoue, MD, Juntendo University School of Medicine, Japan. fwif1005@mb.infoweb.ne.jp (35) Yuko Kaneko, MD, PhD, Division of Rheumatology, Department of Internal Medicine, Keio University School of Medicine, Japan. ykaneko@z6.keio.jp (36) Taku Kawasaki, MD, PhD, Department of Orthopaedic Surgery, Shiga University of Medical Science, Japan. tka@belle.shiga-med.ac.jp (37) Shigeto Kobayashi, MD, Juntendo University School of Medicine, Japan. shigeto@juntendo.ac.jp (38) Manjari Lahiri, MD, Division of Rheumatology, National University Hospital, Singapore. Manjari_lahiri@nuhs.edu.sg. (39) Hernán Maldonado-Ficco, MD. nanmaldonado@msn.com. Clinica Regional del Sud, Rio Cuarto (Córdoba), Argentina. (40) Marhadour, MD, Rheumatology Department, CHRU Cavale Blanche, Brest, France. (41) Alejandro Martínez, MD. ale_mmzgonc@yahoo.com.ar. Hospital Tornú, Buenos Aires, Argentina. (42) Kazuo Matsui, MD, Department of Rheumatology, Kameda Medical Center, Japan. PXU17054@nifty.com (43) Ramón Mazzuchelli Esteban, MD, Hospital Fundación Alcorcón, Madrid, Spain. rmazzucchelli@fhalcorcon.es (44) Corinne Micelli, MD, PhD, Rheumatology Department, Kremlin-Bicêtre Hospital, Paris VII University, Paris, France. (45) Chisun Min, MD, Immuno-Rheumatology Center, St Luke’s International Hospital, Japan. sinrai7055@gmail.com (46) Mitsuhiro Morita, MD, PhD, Department of Orthopaedic Surgery, Fujita Health University, Japan. mimorita@fujita-hu.ac.jp (47) Juan Mulero Mendoza, MD, PhD, Hospital Universitario ‘Puerta de Hierro’, Madrid, Spain. juan.mulero@arrakis.es (48) Jose Raul Noguera Pons, MD, Hospital de Elche, Elche, Spain. noguera_jos@gva.es (49) Masato Okada, MD, Immuno-Rheumatology Center, St Luke’s International Hospital, Japan. okadama@luke.ac.jp (50) Alberto Ortiz, MD. albertoortiz_4@hotmail.com Hospital Provincial Dr. José Cullen, Santa Fe, Argentina. (51) Jon Packham, DM, FRCP, Keele University. Jon.Packham@uhns.nhs.uk (52) Gisela Pendón, MD. giselapendon@yahoo.com.ar. Hospital Ricardo Gutierrez, La Plata, Argentina. (53) Dora Pereira, MD. dorapereira@fibertel.com.ar. Hospital Ricardo Gutiérrez, La Plata, Argentina. (54) José A Pereira da Silva, MD, Clínica Universitária de Reumatologia, Hospitais da Universidade de Coimbra, Coimbra, Portugal. (55) Fernando Pimentel-Santos, MD, Faculdade de Ciências Médicas, Universidade Nova de Lisboa, Lisbon, Portugal. (56) Hanan Rkain, MD, Mohamed Vth University, URAC 30, Department of Rheumatology, El Ayachi Hospital, Salé, Faculty of Medicine and Pharmacy, Rabat, Morocco. hananrkain@yahoo.fr (57) Oscar Rillo, MD. rillo.oscar@gmail.com. Hospital Pirovano, Buenos Aires, Argentina. (58) Carlos Rodriguez Lozano, MD, Hospital Universitario Negrin, Tenerife, Spain. crlormar@gmail.com (59) Adeline Ruyssen-Witrand, MD, PhD, Centre de Rhumatologie, CHU Purpan, Toulouse, France. (60) Adrián Salas, MD. adrianpsalas@yahoo.com.ar. Hospital General San Martín, La Plata. Argentina. (61) Carlos Salinas-Ramos, MD, Escuela Superior de Medicina, Instituto Politécnico Nacional, Mexico. (62) Amelia Santosa, MD, Division of Rheumatology, National University Hospital, Singapore. amelia_santosa@nuhs.edu.sg (63) Alain Saraux, MD, PhD, Rheumatology Department, CHRU Cavale Blanche, Brest, France. (64) Raj Sengupta, FRCP, PGCME, Royal Bath Hospital for Rheumatic Diseases, Bath, UK. rajsen99@gmail.com (65) Stefan Siebert, PhD, University of Glasgow, UK. stefan.siebert@glasgow.ac.uk (66) Martin Soubrier, MD, PhD, CHU Clermont-Ferrand, Dpt of Rheumatology, UMR 1019 INRA/Clermont 1 University, Clermont-Ferrand, France. (67) Caroline Spiegel, Rheumazentrum Ruhrgebiet, Herne, Germany. carolinspiegel@gmx.de (68) Carmen Stolwijk, MD, Maastricht University Medical Center, Maastricht, The Netherlands. (69) Kurisu Tada, MD, Juntendo University School of Medicine, Japan. chris@juntendo.ac.jp (70) Naoho Takizawa, MD, Department of Rheumatology, Kameda Medical Center, Japan. ttkkzzww5959@gmail.com (71) Yoshinori Taniguchi, MD, PhD, Department of Endocrinology, Metabolism, Nephrology and Rheumatology, Kochi University, Japan. taniguchiy@kochi-u.ac.jp (72) Atsuo Taniguchi, MD, PhD, Institute of Rheumatology, Tokyo Women’s Medical University, Japan. amtanigu@ior.twmu.ac.jp (73) Chung Tei Chou, MD, Veterans General Hospital, Taipei, Taiwan. ctchou1007@gmail.com (74) Lay-Keng Teoh, Division of Rheumatology, National University Hospital, Singapore. lay_keng_teoh@nuhs.edu.sg (75) Tetsuya Tomita, MD, PhD, Department of Orthopaedic Biomaterial Science, Osaka University Graduate School of Medicine, Japan. tomita@ort.med.osaka-u.ac.jp (76) Wen-Chan Tsai, MD, PhD, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan; email: d10153@ms14. hinet.net (77) Shigeyoshi Tsuji, MD, PhD, Department of Orthopedic surgery, Osaka Minami Medical Center, Japan. hige.t1970@gmail.com (78) Olga Tsyplenkova, Rheumazentrum Ruhrgebiet, Herne, Germany. carolinspiegel@gmx.de (79) Astrid van Tubergen, MD, PhD, Maastricht University Medical Center, Maastricht, The Netherlands. (80) Kiana Vakil-Gilani, BS, MPH, Oregon Health & Science University, Portland, USA. (81) Rafael Valle-Oñate, MD, Rheumatology Department, Faculty of Medicine, HMC/UMNG, Bogota, Colombia. (82) Gaelle Varkas, MD, Department of Rheumatology, Ghent University Hospital, Ghent, Belgium. (83) Virginia Villaverde, MD, Hospital de Mostoles, Madrid, Spain. virginia.villaverde@yahoo.es (84) Ai Yap, Division of Rheumatology, National University Hospital, Singapore. B.Sc ai_yap@nuhs.edu.sg (85) Pedro Zarco Montejo, MD, PhD. Hospital Fundación Alcorcón, Madrid, Spain. pzarco@fhalcorcon.es.