Article Text
Abstract
Objectives: To evaluate the diagnostic sensitivity of antibodies to cyclic citrullinated peptide (CCP) in recent onset rheumatoid arthritis (RA) at diagnosis and 3 years later, and to evaluate anti-CCP antibody as a predictor of the disease course during 3 years.
Methods: 242 patients with recent onset (⩽1 year) RA were followed up regularly during 3 years after inclusion in the Swedish multicentre study “TIRA” 1996–98. Anti-CCP antibodies were analysed by an enzyme immunoassay (EIA). Rheumatoid factors (RFs) were analysed by latex agglutination and two isotype-specific (IgM and IgA) EIAs. Disease activity was assessed by plasma CRP, ESR, 28 joint disease activity score, and the physician’s global assessment of disease activity. Functional ability was evaluated by the Health Assessment Questionnaire.
Results: Overall, the diagnostic sensitivity of anti-CCP antibodies was 64% and the proportion of positive tests increased with the number of fulfilled classification criteria according to the American College of Rheumatology. The anti-CCP antibody results correlated with RF, but were better than RF as predictor of a more aggressive disease course. After 3 years 5/97 patients had changed anti-CCP status: 2 from negative to positive and 3 from positive to negative. The mean level of anti-CCP antibodies declined by 131 U/ml during the 3 year follow up (95% CI 34 to 228 U/ml).
Conclusion: The anti-CCP antibody assay has a similar diagnostic sensitivity to that of RF in early RA, but is better as a predictor of the disease course over 3 years. Although the mean serum level declines, anti-CCP antibody positivity remains essentially unaltered 3 years after diagnosis and start of antirheumatic treatment.
- ACR, American College of Rheumatology
- CCP, cyclic citrullinated peptide
- COMP, cartilage oligomeric protein
- CRP, C reactive protein
- DAS28, 28 joint count disease activity score
- DMARD, disease modifying antirheumatic drug
- EIA, enzyme immunoassay
- ESR, erythrocyte sedimentation rate
- HAQ, Health Assessment Questionnaire
- PGA, physician’s global assessment
- RA, rheumatoid arthritis
- RF, rheumatoid factor
- anti-CCP antibody
- rheumatoid factor
- early rheumatoid arthritis
- inflammation
- disease course
Statistics from Altmetric.com
- ACR, American College of Rheumatology
- CCP, cyclic citrullinated peptide
- COMP, cartilage oligomeric protein
- CRP, C reactive protein
- DAS28, 28 joint count disease activity score
- DMARD, disease modifying antirheumatic drug
- EIA, enzyme immunoassay
- ESR, erythrocyte sedimentation rate
- HAQ, Health Assessment Questionnaire
- PGA, physician’s global assessment
- RA, rheumatoid arthritis
- RF, rheumatoid factor
Apart from pain and functional impairments, rheumatoid arthritis (RA) is associated with increased comorbidity and mortality, mainly due to coronary heart disease.1–,3 The modern treatment strategy is to institute early aggressive treatment with disease modifying antirheumatic drugs (DMARDs), which has improved disease outcome compared with the old “pyramid approach”.4–,7 Optimisation of early aggressive DMARD treatment, however, demands prompt and accurate diagnosis as well as information of prognostic value. Rheumatoid factor (RF) is widely used for both purposes, although the diagnostic and prognostic information provided has been debated.8,9 Other inflammatory conditions and high age are considered “risk factors” for false positive RF; the sensitivity for diagnosis of RA has been reported to be 50–80% and the specificity 70–80%.10,11
In proteins such as (pro)filaggrin,12 fibrin,13 and vimentin,14 the anti-cyclic citrullinated peptide (anti-CCP) antibody recognises arginine residues modified by peptidylarginine deiminases.15 Anti-CCP belongs to the family of antifilaggrin autoantibodies, accompanied by the antikeratin antibody and the antiperinuclear factor.16 A commercial enzyme immunoassay (EIA), containing a synthetic citrullinated peptide, has been developed for detecting anti-CCP antibodies.17 The second generation CCP2 test, that was used in this study, has been reported to be as specific (90–99%) but more sensitive (66–88%) for RA.18–,23 The extreme diagnostic specificity of anti-CCP for RA raises the question of an aetiopathogenetic connection. Comparisons of the prognostic value of RF and anti-CCP measured as radiological progression have shown varying results, although most studies refer to the less sensitive CCP1 test.24–,26 Little has been done to study the relation of anti-CCP antibody positivity and the clinical disease course over time in early RA. Neither have, to the best of our knowledge, follow up results on anti-CCP antibodies for “seroconversion” or changing serum levels been published. This study was carried out to investigate clinical and laboratory disease activity measures in patients with RA of recent onset in relation to the seropositivity for anti-CCP antibodies, and to explore changes in anti-CCP levels between inclusion and the 3 year follow up.
PATIENTS AND METHODS
Patients and control subjects
Three hundred and twenty patients with early RA were included in the prospective cohort designated TIRA (a Swedish acronym for “early intervention in rheumatoid arthritis”) during 27 months in 1996–98.27 Ten rheumatology centres in southeast Sweden participated. All patients had symptom duration of at least 6 weeks, but less than 12 months. At inclusion, the patients fulfilled either four of seven American College of Rheumatology (ACR) criteria or the following: morning stiffness ⩾60 minutes, symmetrical arthritis and small joint arthritis (metacarpo-/metatarsophalangeal joints/wrists). Serum samples were available for anti-CCP analysis from 242 TIRA patients at inclusion (166 women and 76 men, mean age 55.1 years) and from 121 after 36 months. Serum samples from 80 healthy blood donors (40 women, 40 men) served as controls. The patients were treated with DMARDs, corticosteroids, non-steroidal anti-inflammatory drugs, and analgesics as considered appropriate by the physician.27 Two hundred and thirty five (97%) patients fulfilled four or more of the seven ACR criteria at inclusion.
Erythrocyte sedimentation rate (ESR) and plasma C reactive protein (CRP) were measured at inclusion and after 6, 12, 24, and 36 months. The physician’s global assessment of disease activity (PGA) was estimated without knowledge of anti-CCP status on a five point scale (0–4), where 0 corresponds with no activity and 4 represents high activity. A 28 joint disease activity score (DAS28) was calculated for all patients.28 Functional ability was assessed by the patients using the Swedish version of the Health Assessment Questionnaire (HAQ).29
Laboratory analyses
Anti-CCP antibodies were analysed by EIA (Immunoscan RA CCP2, Euro-Diagnostica, Arnhem, the Netherlands) according to the manufacturer’s instructions. All sera were tested in duplicate and the average results presented. Sera were stored at −72°C until use. The cut off value for positive reaction was set at 25 U/ml as suggested by the manufacturer. The intra-assay variation was 13.6% with a “high level serum” (1360 U/ml) and 6.6% with a “low level serum” (79 U/ml), and the interassay variation was 10.5% and 7.8%, respectively.
Latex particle agglutinating RF was analysed at the laboratories affiliated to the patients’ local hospitals. RFs of IgM and IgA class were analysed by EIA (Autozyme RF IgM and IgA, respectively, Cambridge Life Sciences, Cambridge, UK). Cut off levels for the RF tests were set using the 95th centile of a reference material of 100 healthy blood donors. IgM RF >34 U/ml and IgA RF >15 U/ml were considered positive.
Cartilage oligomeric protein (COMP) was measured by a capture EIA (Anamar Medical, Lund, Sweden).
Statistical analysis
Statistical analysis was performed using SPSS statistical software. The relation between RF and anti-CCP (both expressed as positive or negative), comparison of the frequency of anti-CCP results in subgroups of the TIRA patients, and differences in DMARD treatment were analysed with Fisher’s exact test. Correlation between levels of anti-CCP antibodies and IgM RF, IgA RF, and COMP were tested using Spearman correlation coefficient. Mann-Whitney U test was used to determine statistical differences between anti-CCP positive and anti-CCP negative patients in ESR, CRP, HAQ, DAS28, and PGA. The difference between anti-CCP antibody level at inclusion and at the 3 year follow up was tested with paired t test. Values of p<0.05 were considered significant.
Ethical considerations
The patients gave written informed consent to participate in the study. The study protocol was approved by the local ethics committees of the participating hospitals.
RESULTS
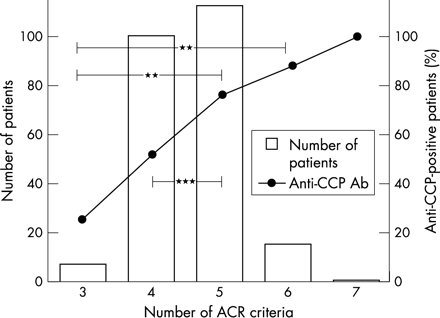
Figure 1⇓ illustrates the anti-CCP antibody results of patients at inclusion in the TIRA study and after 3 years, and in healthy blood donors. For the whole group of patients with RA, the sensitivity of the anti-CCP antibody test was 64% at inclusion and 59% after 3 years, a difference that was not statistically significant. Figure 2⇓ shows that the proportion of anti-CCP antibody positive patients increases with the number of ACR criteria fulfilled. Of the 97% of patients with RA fulfilling ⩾4/7 ACR classification criteria for RA, 67% were anti-CCP positive at inclusion compared with 25% anti-CCP positive patients in the remaining 3% (p<0.05). DMARD treatment was significantly more often started in patients positive for anti-CCP at inclusion than in anti-CCP antibody negative patients. The difference was significant at all visits during the study. Table 1⇓ presents the details of DMARD treatment and differences in prescription rates. Notably, although DMARD treatment in general was more often prescribed to the anti-CCP antibody positive patients, antimalarial drugs were more often prescribed to patients in the anti-CCP antibody negative group (table 1⇓).
Proportion of patients (%) with a positive or negative anti-CCP test at baseline in relation to continuing or prescribed treatment during the study
Anti-CCP antibody levels in the TIRA population at baseline and after 3 years, and in the healthy blood donors.
Frequency distribution of the number of fulfilled classification criteria for RA (ACR 1987) in relation to the proportion of positive anti-CCP antibodies at baseline. **p<0.01; ***p<0.001.
After 3 years’ duration, serum samples from 96 patients were available for comparison with samples obtained at inclusion in the study. In 18 (19%) cases the anti-CCP antibody level had increased >15%, 34 (35%) had decreased >15%, and in 44 (46%) patients the anti-CCP level remained unchanged ±15%. Two of the initially anti-CCP antibody negative patients (2%) had converted to anti-CCP antibody positive, and in three cases (3%) seropositivity had converted to antibody negative. The patients who changed from positive to negative all received DMARD treatment at inclusion, whereas neither of the two patients who converted from negative to positive had been prescribed DMARDs during the first 3 months, although both received DMARDs within the first year. The mean level of anti-CCP antibodies declined (p<0.01) by 131 U/ml during the 3 year follow up (95% confidence interval (CI) 34 to 228 U/ml).
Table 2⇓ illustrates the results for the agglutinating RF and anti-CCP antibody tests, respectively. The sensitivities of agglutinating RF and anti-CCP were both 64% (table 2⇓). Fifty per cent of the patients were positive in both tests (table 2⇓). There was a significant propensity for anti-CCP antibody positive patients to be positive also for RF (p<0.001). Seventy eight per cent of the RF positive and 40% of the RF negative TIRA patients were anti-CCP antibody positive. There was no significant difference between the sexes for the autoantibody results (data not shown).
Cross tabulation of IgG anti-CCP antibody occurrence and agglutinating RF results in recent onset RA
EIA analyses of RF showed that IgM RF occurred in 74%, IgA RF in 72%, and both isotypes in 64% of the patients. The Spearman correlation coefficients for correlation of levels of anti-CCP antibodies with IgM RF and IgA RF, respectively, were 0.52 in both instances, but with R2<0.1, suggesting that only a few data points contributed to the correlation.
Figure 3⇓ illustrates the differences between anti-CCP positive and anti-CCP negative patients in disease activity measures (ESR, CRP, DAS28, number of swollen joints, and PGA) measured at inclusion and after 6, 12, 24, and 36 months’ follow up. ESR was consistently significantly higher in the anti-CCP positive group (n = 103) than in the anti-CCP negative group (n = 63) with p<0.05 at inclusion and 36 months, p<0.01 at 6, 12, and 24 months. CRP was significantly higher in the anti-CCP positive patients (n = 109) than in the anti-CCP negative group (n = 62) at 6 (p<0.001) and at 12 and 24 months (p<0.01). DAS28 was significantly higher in the anti-CCP positive group (n = 86) than in the anti-CCP negative (n = 50) at 12 months (p<0.05). PGA scores were significantly higher among the anti-CCP positive (n = 88) than among the anti-CCP negative patients at baseline, and after 6, 12, and 24 months (p<0.05 at all occasions), whereas at 36 months the difference did not quite reach significance (p = 0.06). HAQ scores showed no significant differences between the groups (data not shown). Efforts to compare the disease course over 3 years (ESR, CRP, DAS28, joint count, and PGA) between the subgroups of patients with rising, unchanged, and declining levels of anti-CRP were inconclusive.
Disease activity measures over 3 years in patients with early arthritis who were anti-CCP positive or anti-CCP negative at diagnosis.
Differences in disease activity measures between IgM RF or IgA RF positive and negative patients showed the same tendency as with anti-CCP. Significantly higher disease activity scores were found in IgM RF positive patients than in IgM RF negative patients for ESR at 6 and 12 months (p<0.05 and p<0.01, respectively), and for CRP at 6, 12, and 24 months (p<0.05, <0.01, and <0.05). There were no significant differences between IgM RF positive and negative patients for PGA, DAS28, or HAQ. For patients with positive IgA RF, ESR was significantly higher at 6 months (p<0.05), CRP at 6 and 12 months (p<0.01 and p<0.05), and PGA at inclusion (p<0.01). There were no significant differences between IgA RF positive/negative patients for DAS28 or HAQ.
No correlations were found between the levels of anti-CCP antibody or RF and serum COMP concentration at inclusion.
DISCUSSION
This study confirms that the diagnostic sensitivity of anti-CCP antibodies in patients with recent onset RA is the same as that of agglutinating RF (64% for both) and that seropositivity for the two tests correlates significantly. In a recent prospective, population based study on very recent onset (<3 months) arthritis, we could also confirm that the anti-CCP test had a diagnostic specificity of 96% for RA as compared with other arthritides.30 The proportion of anti-CCP antibody positive patients increased in parallel with the increasing number of ACR classification criteria. We also found that the readiness to institute DMARDs (without knowledge of anti-CCP status) was significantly higher in the group of anti-CCP positive patients, probably reflecting the PGA of a more aggressive disease in this group of patients than in the anti-CCP antibody negative group. Interestingly, although the use of DMARDs, in general, was greater in the anti-CCP antibody positive patient group, the use of antimalarial drugs as a single DMARD (which can be considered to be a “timid” DMARD strategy) was more common in the anti-CCP antibody negative patient group. A similar proportion of positive anti-CCP antibody results was found 3 years after the diagnosis of RA (59%) compared with baseline (64%), but on average at a significantly decreased level. The number of patients not treated with DMARDs during these years, however, was too small to evaluate any influence of DMARD treatment on the levels of anti-CCP antibodies.
An interesting finding was that anti-CCP antibody positivity at diagnosis predicted higher disease activity over the 3 following years of recent onset RA (fig 3⇑). A similar trend was found for both IgM RF and IgA RF, although not to the same extent. Although controversial,8 it has been claimed that IgA RF directed against human IgG31,32 or rabbit IgG,33 is a better predictor of an aggressive disease course in RA than IgM RF, but this could not be confirmed in our study. However, Houssien et al have pointed out that rabbit IgG is the preferable source of antigen.34
The time course of the patients’ HAQ score showed no major differences between anti-CCP antibody seropositive/negative cases during the study. This may appear to contradict the significant findings related to the disease activity markers ESR, CRP, DAS28, and PGA. However, we believe that this is explained by the fact that HAQ reflects another aspect of the disease—that is, functional ability. Wolfe and Pincus reported that when patients with RA of less than 2 years’ duration were classified according to the quartile of their ESRs, these quartiles were maintained over time.35 They suggested that inflammatory activity is “set” early in RA and remains stable for a long time. In the light of this, we find it interesting that the ESR was consistently higher (as were the levels of CRP and the PGA of disease activity), in anti-CCP antibody positive patients than in the anti-CCP antibody negative cases. Today it is generally agreed that RA should be diagnosed as early as possible and that potent antirheumatic disease modifying treatment should be started as early as possible.6,7 However, ideally the therapeutic strategy should be individually tailored—for example, by distinguishing cases with mild disease and good prognosis from those with severe disease with bad prognosis, in order both to avoid overtreatment with potentially toxic antirheumatic drugs and to start the most aggressive (and costly) treatments for the patients who would benefit most from this.36 To be able to identify “the right patient for the right treatment strategy”, good predictors are needed. Until recently, RF has been the best known laboratory predictor of disease activity and progression of erosive RA.37,38 The recently described anti-CCP antibody assay has already become established as a sensitive and highly disease-specific diagnostic marker of RA. In our study we also found that it was better than RF as a predictor of disease activity over 3 years after the diagnosis of recent onset RA.
We conclude that anti-CCP antibody status at the time of diagnosis of early RA is a valuable predictor of the disease course in early RA. Further studies are needed to evaluate the usefulness of anti-CCP antibody analysis for design of individual therapeutic strategies in early RA.
Acknowledgments
We thank Mrs Ylva Billing and all TIRA partners in Eskilstuna, Jönköping, Kalmar, Lindesberg, Linköping, Motala, Norrköping, Oskarshamn, Västervik, and Örebro for their excellent cooperation.
Grant support: The Swedish Rheumatism Association, The Medical Research County Council of South-East Sweden (FORSS), The Swedish Research Council (project K2003-74VX-14594-01A), the County Council of Östergötland, King Gustaf V 80 year Foundation, The National Board of Health and Welfare.