Article Text
Abstract
Background Anti-citrullinated protein antibodies (ACPA) are the most predictive factor for the development of rheumatoid arthritis (RA).
Objective To investigate whether the recognition of citrullinated epitopes changes during disease onset or progression, by studying the fine specificity of ACPA in serum samples collected throughout the disease course, from before the onset of arthritis to longstanding RA.
Methods Antibodies recognising five distinct citrullinated antigens were determined by enzyme-linked immunosorbent assay. Serum samples from 36 individuals who had donated blood before and after disease manifestation were used to investigate the development of citrullinated antigen recognition before disease onset. The association of ACPA reactivities with disease outcome was studied using sera from anti-cyclic citrullinated peptide-2 (CCP2)-positive patients with undifferentiated arthritis (UA) who did or did not progress to RA (UA–RA n=81, or UA–UA n=35). To investigate the ACPA recognition profile in patients with RA over a prolonged period of time, baseline serum samples from 68 patients were compared with samples obtained 7 years later.
Results The number of recognised citrullinated peptides increased in the period preceding disease onset. At the time of disease manifestation, patients with UA who later developed RA recognised significantly more peptides than UA–UA patients. At later stages of the disease course, the ACPA fine specificity did not change.
Conclusion Epitope spreading with an increase in the recognition of citrullinated antigens occurs before the onset of RA. Immunological differences in ACPA fine specificity between UA–UA patients and UA–RA patients are present at baseline and are associated with the future disease course.
Statistics from Altmetric.com
Introduction
Anti-citrullinated protein antibodies (ACPA) are a very distinctive feature of patients with rheumatoid arthritis (RA). The presence of these antibodies, which is most commonly assessed by reactivity against cyclic citrullinated peptide-2 (CCP2), has been shown to be highly predictive for both the development of RA and the extent of associated joint destruction.1 2 Recent evidence indicates that well-known genetic risk factors for RA: the HLA-DRB1 shared epitope (SE) alleles and the PTPN22 T allele are predominantly associated with ACPA-positive RA.3 4 These reports, together with the finding that ACPA can exacerbate arthritis in mice,5,–,7 suggest that anti-peptidylcitrulline immunity has an important role in the pathogenesis of the disease. To elucidate the biological mechanisms underlying RA, and possibly identify targets for intervention, it is therefore important to gain more insight into the development of ACPA.
Studies investigating at which point in time ACPA first appear have shown that these antibodies can often be detected several years before disease onset.8,–,10 The mere presence of ACPA therefore does not appear to be sufficient to precipitate disease. An explanation for this observation might be be that the anti-citrullinated protein immune response first needs to mature more fully, in the course of which, ACPA may acquire distinct characteristics which are instrumental in mediating tissue damage.
An antibody characteristic which has been shown to be crucial for the pathogenicity of autoantibodies, is the fine specificity of antigen recognition.11 An increase or shift in antigen recognition during the course of an immune response (a phenomenon known as epitope spreading) can have very important pathophysiological consequences as has been described in, for example, systemic lupus erythematosus (SLE).12
Taking into consideration the fact that ACPA can be detected before the clinical diagnosis of RA, and that the presence of ACPA is strongly associated with disease progression, we hypothesised that epitope spreading of the ACPA response may have a role in the evolution of the disease. In this study we therefore investigated the reactivity pattern of ACPA before disease onset and during disease progression. In order to cover the entire spectrum of the disease course, ranging from ACPA-positive healthy individuals to patients with longstanding RA, we made use of several different cohorts, which together provide an overview of the ACPA fine-specificity development over time.
Materials and methods
Study population
In a previous study, individuals who had donated blood before the onset of arthritis were identified among patients with RA treated at the Department of Rheumatology of the Umeå University Hospital.8 The time period between collection of the predisease sera and disease onset ranged from 43 days to 10.8 years, with a median of 2.5 years.
Patients with undifferentiated arthritis (UA) or RA were selected from the Leiden Early Arthritis Clinic, an inception cohort of patients with recent-onset arthritis (complaints for <2 years) that was initiated at the Department of Rheumatology of Leiden University Medical Centre in 1993.13 Diagnoses were recorded for all patients at annual follow-up visits and RA was diagnosed according to the 1987 revised American College of Rheumatology criteria. Patients who did not fulfil the diagnosis criteria and whose clinical presentation was not compatible with any other well-defined rheumatological disease entity, were classified as having UA. For this study we analysed 116 anti-CCP-positive patients with UA and 68 anti-CCP-positive patients with RA.
Informed consent was obtained and the study was approved by the local medical ethics review board.
Anti-CCP2 assays
Total IgG anti-CCP2 was measured by enzyme-linked immunosorbent assay (ELISA) (Immunoscan RA Mark 2; Eurodiagnostica, Arnhem, The Netherlands). Samples with a value >25 U/ml were considered positive according to the manufacturer's instructions.
Anti-citrullinated peptide assays
ELISA assays were developed against peptides derived from vimentin, fibrinogen and α-enolase. Although there are also other targets for ACPA,14 15 we chose primarily to investigate epitopes from these proteins because they have been most consistently identified as citrullinated autoantigens.16,–,18
Antibodies against both the citrullinated (Cit) and the uncitrullinated form of two linear peptides derived from vimentin (Vim 1–16: STCitS VSSS SYCitCit MFGG and Vim 59–74: VYAT CitSSA VCitLCit SSVP), two linear peptides derived from fibrinogen (Fibα 27–43: FLAE GGGV CitGPR VVER H and Fibβ 36–52: NEEG FFSA CitGHR PLDK K) and one linear peptide derived from α-enolase (Eno 5–20: KIHA CitEIF DSCitG NPTV) were determined by ELISA. The vimentin and fibrinogen epitopes used for this study were selected because they were most frequently recognised by sera from ACPA-positive patients with longstanding RA. A linear α-enolase peptide was used with a small difference in sequence compared with the peptide used by Lundberg et al.19 Because antibodies from different patients with RA have been shown to recognise distinct epitopes of the same protein, an approach using citrullinated peptides rather than whole proteins was used in order to attain optimal discriminative ability.20
Fine-specificity ELISA assays were performed as described previously.21 Briefly, streptavidin-coated preblocked microtitre plates were coated with the different peptides, followed by incubation with the serum samples. After washing, antibodies were detected with a rabbit anti-human IgG horseradish peroxidase-conjugated antibody and tetramethylbenzidine as the colouring substrate. Baseline and follow-up samples were always analysed on the same day and on the same plate. The interassay variability of the assays was very low (≤2% of samples with conflicting positive/negative results).
Definition of cut-off values and citrulline specificity
Cut-off values on the citrullinated and arginine-containing peptides were defined as the mean plus 2 SD of the values of 30 control subjects. A patient sample was considered to recognise a particular peptide in a citrulline-specific manner when it fulfilled all three of the following requirements: (1) an optical density (OD) value on the citrullinated peptide above the citrulline cut-off point; (2) an OD value on the arginine variant below the arginine cut-off point; (3) an OD difference (=OD for citrullinated peptide – OD for arginine-containing peptide) of at least 0.1. The number of patients who recognised both the citrullinated and the arginine-containing peptide above cut-off levels and thus did not bind to the peptide in a citrulline-specific way was small (approximately 3%).
Statistical analysis
To assess changes in the frequency of recognition of the citrullinated peptides between baseline and follow-up samples, McNemar's test was used. Differences in the number of epitopes recognised, and in the levels of antibody reactivity between baseline and follow-up samples were evaluated using Wilcoxon signed ranks test. When two independent patient groups were compared (UA–UA vs UA–RA patients), Mann–Whitney U tests were used to analyse differences between median OD values and the median number of recognised peptides. Relative risks (RRs) with 95% CI were calculated for the development of RA from UA, based on the presence of a certain fine specificity. Logistic regression analysis was performed to calculate the risk associated with recognising one additional citrullinated peptide for the outcome UA or RA after 1 year of follow-up. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) 14.0 and StatCalc 2.0.
Results
Epitope spreading occurs before disease onset
In order to assess the recognition of citrullinated epitopes before disease onset, we made use of a unique serum collection of 36 individuals who had donated blood before the onset of RA and shortly after disease manifestation (on average 7.5 months after disease onset).8 As shown in figure 1A, the serum samples collected before disease onset (pre-RA) had a significantly lower reactivity against all of the peptides, except for vimentin 1–16, than the sera collected after disease manifestation. For individual patients, the changes in reactivity to the different peptides over time were often correlated, but this correlation was not absolute.
(A) Reactivity of serum samples collected before disease onset (pre-rheumatoid arthritis (pre-RA)) and after disease onset (RA). The number of U/ml against cyclic citrullinated peptide-2 and the raw optical density (OD) values against the five fine-specificity peptides are shown for the 36 individuals from whom serum was collected before and after disease onset. Differences between the median levels at the pre-RA and RA time points against each of the five peptides were investigated by Wilcoxon signed rank tests. CCP, cyclic citrullinated peptide; Eno, enolase; Fibα/β, fibrinogen α/β; Vim, vimentin. (B) Differences in peptide recognition between samples obtained before disease onset (pre-RA) and after disease onset (RA). The percentages of pre-RA and RA sera (n=36) that bound to the indicated number of peptides are shown. (C) Development of epitope recognition before the onset of arthritis. The median number of peptides recognised by serum samples available from the specified time period antedating the symptoms of RA (n=36) and at the time RA was diagnosed (n=36) is depicted.
Most peptides, with the exception of vimentin 1–16, were recognised substantially more frequently by the RA samples than by the pre-RA sera as listed in table 1. The presence of (one or more isotypes of) rheumatoid factor (RF) also increased in the period before disease onset (data not shown). While many of the sera demonstrating reactivity to one of the fine-specificity peptides also contained IgA-RF or IgM-RF, this was not always the case. Recognition of the fine-specificity peptides was limited to the 29 individuals who were anti-CCP2-positive after disease onset.
Recognition of individual citrullinated peptides before and after disease onset
The overall gain in peptide recognition resulted in a significant increase in the number of patients who recognised one or more peptides (38% vs 66%, p=0.01) (figure 1B). The median number of recognised peptides was zero before disease development compared with one in the RA samples (p=0.08). As the period between pre-RA sample collection and disease manifestation varied among patients, the expansion of epitope recognition over time is shown in more detail in figure 1C. This figure illustrates that the recognition of citrullinated epitopes increases before disease onset.
Taken as a whole, the analysis of the ACPA fine-specificity profile before and after disease onset shows that epitope spreading occurs before the onset of RA.
The baseline ACPA reactivity profile differs between patients with UA who progress to RA (UA–RA) and those who do not (UA–UA)
To investigate if a distinct recognition pattern of citrullinated peptides is associated with disease progression, we made use of serum samples from a different cohort: the Leiden Early Arthritis Clinic. ACPA reactivities were determined in baseline serum samples from patients with UA who had not been treated with disease-modifying antirheumatic drugs (DMARDs). Of 116 anti-CCP2 positive patients with UA, 81 fulfilled the American College of Rheumatology criteria for RA after 1 year of follow-up (UA–RA), whereas the remaining 35 patients did not develop RA (UA–UA). In figure 2A, the baseline reactivity is depicted in the form of the raw OD values. At baseline, the reactivity of the UA–RA sera was significantly higher than that of the UA–UA sera against two of the five peptides. When the OD values were translated into negative/positive recognition by applying cut-off values based on 30 control subjects, the prevalence of recognition of both vimentin peptides and fibrinogen β 36–52 was significantly higher among UA–RA than among UA–UA patients (figure 2B). As shown in figure 2C, the median number of recognised peptides per patient was larger in UA–RA patients (two peptides) than in UA–UA patients (one peptide) (p=0.02). Regression analysis showed that for every additional epitope which was recognised, the risk of developing RA increased by a factor of 1.55 (p=0.02).
(A) Baseline reactivity of UA–UA vs UA–RA sera. Depicted are the raw optical density (OD) values for each of the UA–UA (n=35) and UA–RA (n=81) patients. Lines indicate the median OD. Differences between the median OD values of the UA–UA and UA–RA patients for each of the five peptides were investigated by Mann–Whitney U tests. (B) Recognition of individual citrullinated peptides at baseline by UA–UA and UA–RA patients. Depicted are the percentages of patients in each group that recognised the different peptides in a citrulline-specific manner. Relative risks with 95% CI were calculated for the development of RA from UA, based on the presence of a certain fine specificity. (C) Number of peptides recognised by UA–UA and UA–RA patients. Error bars indicate the minimum and maximum number of peptides recognised per patient. Boxes designate the 25th and 75th centiles, while the lines in the boxes indicate the median number of recognised peptides. Eno, enolase; Fibα/β, fibrinogen α/β; RA, rheumatoid arthritis; UA, undifferentiated arthritis; Vim, vimentin.
In summary, these results indicate that ACPA-positive UA–UA and UA–RA patients are immunologically distinct at baseline with regards to their ACPA reactivity profile. The UA–RA patients displayed reactivity against a significantly larger number of citrullinated epitopes.
Recognition of citrullinated peptides does not change during disease progression
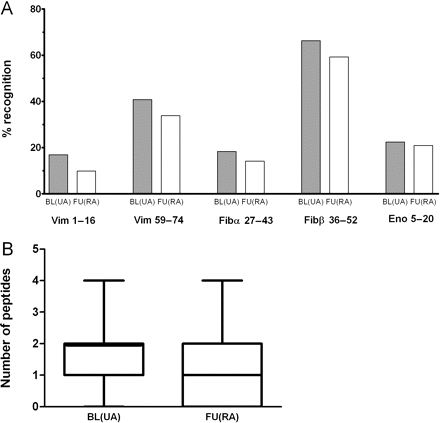
To investigate whether the reactivity against citrullinated antigens would further increase during disease development from UA to RA, we performed fine-specificity assays with serum samples obtained at baseline and after approximately 1.5 years of follow-up of the same UA–RA patients as described above. All patients had progressed to RA at this point in time. Serum samples were available for 67 of the previously tested 81 patients. As shown in figure 3A, the recognition of all five fine-specificity peptides did not markedly change. At follow-up, a smaller number of patients recognised the individual peptides, but the difference was not statistically significant. The number of citrullinated peptides recognised by each patient was also lower at follow-up than at baseline (figure 3B).
(A) Recognition of individual citrullinated peptides by UA–RA patients at baseline and after 1.5 years of follow-up. Depicted are the percentages of patients (n=67) which recognised the peptides in a citrulline-specific manner at baseline (when all patients had UA), and after 1.5 years of follow-up when all patients had developed RA. (B) Number of peptides recognised by UA–RA patients at baseline and after 1.5 years of follow-up. Error bars indicate the minimum and maximum number of peptides recognised per patient. Boxes designate the 25th and 75th centiles, while the lines in the boxes indicate the median number of recognised peptides. BL, baseline; Eno, enolase; Fibα/β, fibrinogen α/β; FU, follow-up; RA, rheumatoid arthritis; UA, undifferentiated arthritis; Vim, vimentin.
On the basis of these results, we can conclude that the ACPA reactivity with regard to the five fine-specificity peptides we measured, did not further increase during the progression of UA to RA.
No changes in recognition of anti-citrullinated peptides by patients with RA after 7 years of follow-up
To further substantiate the finding that the ACPA recognition pattern does not change during the course of the disease, we examined a group of 68 patients who presented with RA and for whom serum samples were available at baseline and after a median follow-up time of 7 years (IQR: 6.2–7.9 years). As depicted in figure 4A, the percentage of patients that recognised the five peptides did not change after 7 years of follow-up. With regard to the absolute number of peptides that was recognised per patient, the findings were similar to the observations described above for UA–RA patients. The number of recognised peptides was slightly lower at the time of follow-up, as can be seen in figure 4B, but this differences was not statistically significant (p=0.25).
(A) Citrulline-specific recognition of the different peptides at baseline and after 7 years of follow-up in 68 patients with rheumatoid arthritis (RA). The percentage of patient samples which bound to the selected peptides citrulline specifically at baseline and after a median of 7 years of follow-up is shown. (B) Number of peptides recognised by 68 patients with RA at baseline and after 7 years of follow-up. Error bars indicate the minimum and maximum number of peptides recognised per patient. Boxes designate the 25th and 75th centiles, while the lines in the boxes indicate the median number of recognised peptides. BL, baseline; Eno, enolase; Fibα/β, fibrinogen α/β; FU, follow-up; Vim, vimentin.
Overall, these results show that for these five fine-specificity peptides, the ACPA recognition pattern does not change during the course of RA and the reactivity profile does not expand after disease onset.
Discussion
In this study, we analysed the recognition pattern of ACPA throughout the disease course of RA. The results show that the recognition of citrullinated epitopes expands before disease onset. Furthermore, at the time of disease onset, an extensive recognition pattern of citrullinated peptides by patients who present with UA is associated with rapid disease progression to RA in the first year of follow-up. After disease development, we could not detect a further increase in reactivity of the five fine-specificity peptides used in this study.
The accumulation of autoantibody reactivities before disease manifestation which we observed in patients with RA, has also been reported in other studies of human disease, such as SLE12 and pemphigus,22 as well as in animal models such as the collagen-induced arthritis model.23 Together, these findings suggest that epitope spreading before disease onset may be a commonly occurring phenomenon which, as shown for the first time by the data presented here, may also take place before the onset of RA.
It is intriguing that at the time of disease onset, ACPA-positive patients with UA who will subsequently develop RA and those who will not, already have an immunologically distinct ACPA response. The fact that none of the tested fine-specificity peptides was exclusively recognised by UA–RA patients indicates that measurement of these ACPA reactivities may not have immediate clinical utility for individual patients. Rather, the results indicate that on a population level, the ‘footprint’ of past epitope spreading (being an expanded epitope recognition repertoire) is associated with disease progression. These differences in the ACPA fine-specificity repertoire are not an isolated immunological phenomenon, since patients with UA who rapidly progress to RA also exhibit a broader use of ACPA isotypes.24 The characteristics of the ACPA response at baseline thus reflect the progression of the disease in patients with UA. These findings are consistent with the hypothesis that the nature and characteristics of the ACPA response may determine disease development and progression, but additional studies will be required to fully elucidate the role of ACPA in the pathogenesis of the disease.
Although baseline sera from UA–RA patients recognised more peptides than sera from UA–UA patients, we could not detect a further increase in reactivity after disease onset. A very similar observation has been reported in SLE, where the accrual of new types of autoantibodies gradually increased up to the time of diagnosis and then virtually stopped.12 These findings are also in line with previous reports which have shown that anti-CCP2 titres and levels of ACPA isotypes decrease with time.24 25 A possible explanation for these observations is the effect of immunosuppressive treatment such as DMARDs, which are commonly prescribed once the diagnosis of RA has been established. This makes it difficult to discern if the observed decrease in ACPA reactivities over time is due to the natural course of the immune response or to the influence of medication.
The patients studied in this investigation were generally given monotherapy with conventional DMARDs. Consistent with the severe phenotype known to be associated with ACPA-positive disease, medication was frequently changed in all patients, which hampered a thorough analysis of clinical subgroups stratified for treatment. Further studies will be required to definitively answer the question of whether specific treatments do or do not affect epitope recognition profiles.
Although we could not observe a further expansion of the ACPA reactivity profile after disease manifestation, it should be noted that the number of citrullinated epitopes investigated in this study is limited. Therefore, one cannot rule out the possibility that epitope spreading after disease onset was in fact present, but could not be detected. However, the data of the cohort of individuals who had donated blood before disease onset show that there is clearly epitope spreading of the ACPA response before disease onset, suggesting that the five peptides had sufficient discriminative potential to allow the detection of diversification of epitope recognition. Nonetheless, further studies using a larger set of citrullinated antigens will be required to fully explore the possibility of epitope spreading after disease onset in RA.
When investigating epitope spreading, two other immunological phenomena must be taken into account: antibody cross-reactivity and affinity maturation. For the peptides used in this study, a previous report described that the HLA SE alleles are strongly associated with the recognition of citrullinated vimentin 59–74, but not with the recognition of citrullinated fibrinogen β 36–52, indicating that there was little cross-reactivity between the different epitopes.21 The fine specificity of the ACPA response, as investigated in our study, was also associated with the presence of HLA SE alleles (data not shown). Furthermore, in this study several patients were single-positive for each specific peptide, which rules out a large extent of antibody cross-reactivity. Affinity maturation, which leads to a higher antibody binding affinity and thus makes antibodies easier to detect by ELISA, could in this manner, have contributed to the increase in epitope recognition which we measured using this method. For our understanding of the immune response in vivo, however, the distinction between epitope spreading and affinity maturation is, in this case, largely inconsequential, as the effect would be the same—namely, increased antibody reactivity to a certain citrullinated epitope.
The in vitro methodology employed in this study cannot mimic the way in which antigen is presented in vivo and was therefore not intended as a technique by which to identify a specific ACPA fine specificity as the culprit of disease onset or exacerbation. Instead, the study was designed to assess the extent to which epitope spreading occurs in association with disease progression by using a selected set of peptides as a model for ACPA diversification. It was not possible to discern clusters of peptides which were preferentially recognised together, nor was it possible to identify a specific sequence in which the ACPA reactivities developed over time.
In summary, our findings indicate that the recognition of citrullinated epitopes expands before the development of arthritis. Furthermore, at the time of clinical presentation, patients with UA who will later develop RA already recognise more citrullinated antigens than patients with UA who will not develop RA. During disease progression from UA to RA, as well as during the further disease course of RA, we did not observe additional changes in anti-citrulline reactivity. Together, these data indicate that an important part of the evolution of the ACPA response takes place before disease onset.
References
Supplementary materials
Web Only Data ard.2009.124537
Files in this Data Supplement:
Footnotes
-
Funding DvdW was supported by the Dutch Organisation for Scientific Research (AGIKO grant). REMT was supported by the Dutch Organisation for Scientific Research (VIDI and VICI grant). Supported in part by research funding from the European Community FP6 funding project Autocure and the FP7 funding project Masterswitch.
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the medical ethical committee university of Umea, and Leiden University Medical Centre.
-
Provenance and peer review Not commissioned; externally peer reviewed.