Article Text
Abstract
Objective To investigate the long-term effects of induction therapy with adalimumab (ADA) plus methotrexate (MTX) in comparison with placebo (PBO) plus MTX in DMARD-naïve patients with active early rheumatoid arthritis (RA).
Methods Patients with active early RA (disease duration of ≤12 months) were randomly assigned to receive 40 mg ADA subcutaneously every other week (eow) plus MTX 15 mg/week subcutaneously or PBO plus MTX subcutaneously at 15 mg/week over 24 weeks. Thereafter, all patients received MTX monotherapy up to week 48. The primary outcome was the Disease Activity Score 28 (DAS28) at week 48. Secondary outcomes included proportions of patients in remission (DAS28<2.6), ACR responses, Health Assessment Questionnaire (HAQ) score and radiographic progression.
Results 87 patients were assigned to ADA/MTX and 85 patients to PBO/MTX. At baseline, DAS28 was 6.2±0.8 in the ADA/MTX and 6.3±0.9 in the PBO/MTX groups. At week 24, treatment with ADA/MTX compared with PBO/MTX resulted in a greater reduction in DAS28 (3.0±1.2 vs 3.6±1.4; p=0.009) and other secondary outcomes such as DAS28 remission rate (47.9% vs 29.5%; p=0.021) and HAQ (0.49±0.6 vs 0.72±0.6; p=0.0014). At week 48, the difference in clinical outcomes between groups was not statistically significant (DAS28: 3.2±1.4 vs 3.4±1.6; p=0.41). Radiographic progression at week 48 was significantly greater in patients administered PBO/MTX (Sharp/van der Heijde score: ADA/MTX 2.6 vs PBO/MTX 6.4; p=0.03, Ratingen score: 1.7 vs 4.2; p=0.01).
Conclusions A greater reduction in radiographic progression after initial combination therapy with ADA and MTX was seen at week 48, even after discontinuation of ADA treatment at week 24. This sustained effect was not found at the primary endpoint (DAS28 reduction).
Statistics from Altmetric.com
Introduction
In patients with early rheumatoid arthritis (RA), irreversible joint destruction starts within the first year.1 After 2 years, 70% of patients show radiographic progression.2 In addition to irreversible function loss, RA patients have a shorter life expectancy compared to the general population.3–5 The concept of a ‘window of opportunity’ describes a period of time when the underlying inflammatory process is more susceptible to drug influences than at later time-points.5–7 In this early phase, the pathogenic and mechanical aspects of autoimmune induced inflammation are not yet fully established.8 Therefore, national and international practice guidelines recommend starting immediate treatment with conventional disease-modifying antirheumatic drugs (DMARDs) and glucocorticoids (GC).8–11
With these shifts in medical therapy, attention is now focused on (very) early disease with the aim of developing treatment regimens that induce long-term suppression of synovitis, prevent joint damage and preserve function, or even stop the disease starting.14–17 Meanwhile, long-term safety data demonstrate a good tolerability profile; however, the direct costs of treatment (10–20-fold higher than traditional DMARDs) are substantial.18–20
Although the initiating events causing RA are still unknown, immunological research has shown that even at the very beginning of an inflammatory cascade the cytokine TNF-α is of crucial importance by stimulating a variety of cells and thus promoting inflammation (eg, the extent of proliferation and the resulting cellular infiltration into the inflamed synovia).21 ,22 In light of the particular features of RA pathogenesis in the early period, TNF-α antagonists including adalimumab (ADA) are likely to prevent the cascade of inflammation prior to joint destruction. The HIT HARD (High Induction Therapy with Anti-Rheumatic Drugs) trial, therefore, introduced the new option of targeted therapy exploiting the immunopathology of the stepwise development of inflammation in RA and the pharmaco-epidemiological postulate of a ‘window of opportunity’ in early RA. The possible superior efficacy of an induction therapy targeting cytokines in early RA, compared to early DMARD therapy, could potentially limit the burden of disease. The aim of this study was to investigate whether or not early treatment with TNF-α antagonists within the ‘window of opportunity’ as induction therapy leads to a long lasting effect that can be maintained when the biologic is withdrawn.
Methods
Trial design
The double-blind randomised controlled trial (RCT) HIT HARD was designed to compare two treatment strategies in DMARD-naive patients with early RA. This multicentre trial was carried out between June 2007 and September 2010 by rheumatologists throughout Germany (private rheumatology practices, hospitals and university departments). An independent data safety monitoring board of external medical expert consultants regularly reviewed the safety and progress of the study. A central institutional review board (Berlin state ethic committee (Landesethikkommission Berlin)) and independent ethics committee at each participating site approved the study, and all patients provided written informed consent.
Participants
Eligible patients were aged 18–75 years, with RA diagnosed according to the revised 1987 American College of Rheumatology (ACR) criteria for the classification of RA, and disease duration of up to 1 year.23 Additional inclusion criteria were at least six joints swollen out of 66, at least six joints tender out of 68, morning stiffness lasting ≥30 min, an erythrocyte sedimentation rate (ESR) of ≥28 mm/h or a C reactive protein (CRP) concentration of ≥1.0 mg/dl. No current or prior therapy with DMARDs or biologics was allowed. Non-steroidal anti-inflammatory drugs (NSAIDs) and GC treatment had to be stable for at least 2 weeks prior to screening and during the trial with a maximum of ≤10 mg/day prednisolone equivalent. Patients were screened for tuberculosis (TB) prior to receiving study drugs (with a purified protein derivative (PPD) and by chest radiography) according to the recommendations of the German Society of Rheumatology (DGRH) at that time. Patients with latent TB were pre-emptively treated with isoniazid (INH; up to 300 mg/day for 9 months).
Exclusion criteria included significant concurrent medical disease, active infection, immunodeficiency, rheumatic disease other than RA, or demyelinating events. Patients were also excluded if they had received previous treatment with other DMARDs, experimental drugs, immunosuppressive drugs, or any live vaccines within 4 weeks of screening, or if they had received intra-articular injections of GC (>10 mg prednisone or equivalent) within 4 weeks.
Interventions
Patients were randomised 1:1 into two treatment groups: blinded ADA 40 mg subcutaneously every other week (eow) or placebo (PBO) subcutaneously eow over 24 weeks; all patients in both groups received open label subcutaneous methotrexate (MTX) weekly (15 mg/week). Thereafter, ADA and PBO injections were stopped, and all patients continued MTX as monotherapy after week 24. In addition, all subjects received folic acid supplementation of 10 mg weekly (24 h after MTX). A stable dose of ≤10 mg/day of prednisone or equivalent was permitted. Patients dropping out of the study received standard of care as decided by the physician.
Outcomes
The (screening and baseline) visit included the following: physical examination including parameters to determine the Disease Activity Score (DAS28ESR) with tender (TJC) and swollen joint count (SJC); the Health Assessment Questionnaire (HAQ); the Short-Form Health Survey with 36 questions (SF-36); laboratory tests measuring ESR, CRP, rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) and safety parameters such as blood cell count, liver and kidney values; the PPD test; chest-x-ray; and radiographs of the hands and feet according to standard of care.
Patients were evaluated every 8 weeks up to week 48 with physical examination for DAS28 calculation, laboratory tests (ESR, CRP, and safety parameters as above), and full 66/68 joint counts. HAQ, SF-36, RF, and ACPA assessments were conducted at weeks 24 and 48.
The primary endpoint was the DAS28 score at week 48 with DAS28 at week 24 as a secondary endpoint. Regarding signs and symptoms, the following secondary endpoints were investigated at week 24 and at week 48: percentage of patients achieving remission (DAS28 <2.6) and percentage of patients achieving ACR20, ACR50 or ACR70 responses.24–26 Change from baseline in Sharp/van der Heijde score (SHS) score was assessed to evaluate inhibition of progression of structural joint damage.27 In addition, all radiographs were assessed using the Ratingen score.28 Radiographs of the hands (posteroanterior view) and feet (anteroposterior view) were obtained and digitised for blinded reading at baseline and at week 48. An experienced reader scored all radiographs in a concealed order.
Sample size
Based on the results of St Clair et al,12 an effect size of 0.5 in DAS28 scores was considered to be achievable and clinically relevant in the comparison of a TNF-α antagonist with an active comparator (MTX) in recent onset RA. A sample size of n=172 was needed to detect a difference in the DAS28 scores which corresponds to an effect size of 0.5 or higher with a power of 90%.
Statistical methods
The statistical analysis was based on all randomised patients. This intention-to-treat population consisted of 172 patients. Analysis of covariance (ANCOVA) with baseline status of the outcome parameter as a co-variable was used to test group differences in DAS28, HAQ and SF-36 scores, TJC and SJC. Non-parametric ANCOVA with baseline radiographic score as co-variable was applied to compare radiographic progression between groups. ESR and CRP values were compared by means of the Mann–Whitney–Wilcoxon test.
Within both treatment groups, the last valid DAS28 score of the non-completers was compared with the mean DAS28 score of the completers at that point in time. 95% CIs of the differences were calculated.29 According to the study protocol, multiple imputations based on regression techniques were used to replace missing values. This method controls for a possible bias caused by non-completers30 and was therefore applied to all outcome parameters except for radiographic outcome measures. Since a dropout rate of 33% was observed in one treatment group, a second statistical approach, the direct likelihood method based on mixed models, was applied in an additional sensitivity analysis. As this approach produced identical results, the results of this second approach are reported only for the primary outcome. Missing radiographic data were not replaced, but patients with and without complete radiographs were compared by means of a mixed models approach. p Values of <0.05 were considered to be statistically significant.
Results
Patient and disease parameters at baseline
A total of 179 patients were assessed for eligibility. Seven patients were excluded as they did not meet the inclusion and/or exclusion criteria. This resulted in 172 patients who were randomly assigned to two groups: 87 patients were assigned to combination therapy with 40 mg ADA subcutaneously eow and 15 mg MTX subcutaneously weekly (ADA/MTX) and 85 patients were given PBO subcutaneously eow and 15 mg MTX subcutaneously weekly (PBO/MTX) from baseline to week 24 (figure 1). After week 24, combination therapy was stopped in both groups, and the patients were subsequently treated only with MTX subcutaneously weekly as monotherapy up to week 48. Overall, 76 patients (87.4%) receiving ADA/MTX and 57 patients (67.1%) receiving PBO/MTX completed 48 weeks of treatment. Patients from the PBO/MTX group who were withdrawn or dropped out had significantly higher disease activity at their last visit compared to the completers of this group at this point in time (mean difference in DAS28 scores 0.90; 95% CI 0.4 to 1.4). This difference was lower in the ADA/MTX group at 0.67 (95% CI 0.0 to 1.3). This confounding by non-completers was taken into account by using an intent-to-treat approach (see Statistics section).
Flow chart of trial of high induction therapy with anti-rheumatic drugs. eow, every other week; s.c., subcutaneously.
Treatment groups were well balanced regarding most demographic and disease characteristics of the patients at baseline. However, characteristics showed significant differences at baseline, for example, mean age, SF-36 physical score and SHS joint space narrowing score (table 1). Moreover, 86.6% of the study patients had symptom duration of <4 months. All patients had active disease with mean DAS28 scores of 6.2±0.86 (ADA/MTX) and 6.3±0.9 (PBO/MTX).
Baseline demographic and disease characteristics
Disease activity and remission
Adjusted for baseline, DAS28 scores differed at week 24 by 0.53 (95% CI 0.13 to 0.93; p=0.009) and HAQ scores by 0.26 (p=0.001) (table 2). Remission rates, SJC, TJC and ACR70 response rates were significantly higher in the ADA/MTX group. After termination of the study drugs (ADA and PBO) and continuation with MTX alone, the differences between the groups decreased.
Comparison of clinical parameters at week 24 and week 48
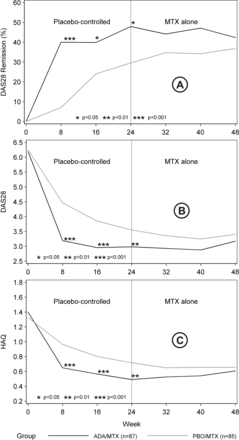
Regarding the primary end point (DAS28 at week 48) no significant differences were found between the groups of patients who started treatment with ADA/MTX combination therapy versus those who started with PBO/MTX. The difference in DAS28 scores between the groups at week 48 was 0.21 (95% CI −0.3 to 0.7; p=0.41) according to the primary statistical approach and 0.17 (95% CI −0.3 to 0.7; p=0.50) according to the secondary statistical approach (see Statistics section; figure 2A). A similar decrease in the differences between the groups was observed in ACR response rates, remission rates (figure 2B), HAQ scores (figure 2C) and physical SF-36 component score (table 2).
(A) DAS28 remission (%); (B) Mean Disease Activity Score in 28 joints (DAS28); (C) mean Health Assessment Questionnaire (HAQ) scores. ADA, adalimumab; MTX, methotrexate; PBO, placebo.
Radiographic progression
Complete radiographic data were available for 51 (59%) patients in the ADA/MTX group and 47 (55%) in the PBO/MTX group (table 1). In these patients, induction treatment with ADA/MTX was significant superior at week 48 compared with PBO/MTX (figure 3A–C). Taking radiographic status at baseline into account, radiographic progression was significantly more pronounced in patients who had started treatment with PBO/MTX: SHS erosion score as compared with the combination group p=0.010, joint space narrowing score p=0.035 (figure 3A), SHS total score p=0.003 (figure 3B) and Ratingen score p=0.012 (figure 3C).
(A) Change in Sharp/van der Heijde score (SHS) erosion score; (B) change in SHS joint space narrowing (JSN) score; (C) change in total SHS. ADA, adalimumab; MTX, methotrexate; PBO, placebo.
PBO/MTX patients with missing radiographs (because they dropped out) had significantly higher mean DAS28 scores of 4.0 (95% CI 3.5 to 4.4) between week 8 and week 48 than those with complete radiographs, whose mean score was 3.4 (95% CI 3.0 to 3.8). This difference was lower and did not reach statistical significance (DAS28 score 3.2; 95% CI 2.9 to 3.6 vs DAS28 score 2.9; 95% CI 2.5 to 3.2) in patients in the combination therapy group. Since radiographic progression was correlated with mean DAS28 scores after the start of treatment (SHS erosion score p=0.04, Ratingen score p=0.09) we may have underestimated radiographic progression in patients who started treatment with PBO/MTX. There was a tendency for more rapid progression in ACPA (but not RF)-positive patients (p<0.08 for the SHS erosion score and p<0.06 for the Ratingen score).
Safety
The trial data analysed revealed that a serious adverse event occurred in 12 (13.7%) of the 87 patients who had received at least one dose of ADA/MTX compared with 22 (19.5%) of the 85 patients in the control group (table 3 and the online supplementary data). The number of serious infections was low (three in the ADA/MTX group and four in the PBO/MTX group). Two patients with PBO/MTX experienced urosepsis (nephrolithiasis in one individual and following biopsy of the prostate in the other), one patient had a paresthesia due to neuralgia of the trigeminal nerve, and one patient experienced diplopia due to paresis of the abducens nerve in diabetes mellitus (both with PBO/MTX). One prostate carcinoma and one cervix carcinoma and a carcinoma in situ of the cervix occurred in the PBO/MTX group. Three patients were hospitalised because of increased disease activity. No cases of tuberculosis or death were observed during the trial in either group.
Serious adverse events
Discussion
The HIT HARD study is the first investigator-initiated blinded multicentre RCT comparing initial 24-week induction therapy with MTX/TNF inhibitor combination therapy with MTX monotherapy in early active MTX-naive RA patients. A similar approach has been taken in the GUEPARD trial, but using 12 weeks of induction in an open design13 and showing no clinical and radiological differences between groups. The objective of our investigation was to determine whether prolonged (24-week) induction therapy would lead to a better outcome after 1 year of overall treatment in a classic RCT. While the response was pronounced at the end of the combination treatment period, this was no longer evident after 1 year with regard to clinical signs. However, the result was significantly better in relation to reduction in radiographic progression.
A paradigm shift has occurred in the treatment of early RA which is now frequently perceived as a medical emergency. Thus, a new approach has evolved for the treatment of RA: early and to target treatment. This approach is based on observations of favourable outcome with early treatment and comparison of early versus delayed therapy in early disease. Also treat to target strategies31 have shown superior results compared to routine approaches.
Thus, intensive treatment usually in combination with low dose GC early in the disease course is evolving with the recognition that early DMARD therapy and subsequent or concomitant combination with biologics improve long-term outcomes. Another aspect of early intervention is the heterogeneity of outcomes in the very early stages of RA, which range from remission to persistent synovitis, either with or without erosive disease. Ideally, the intensity of DMARD therapy should be tailored to the potential severity of disease to maximise the benefit–risk ratio. However, prognostic markers are not yet sufficiently predictive in early disease to be clinically useful on an individual basis. Serological markers, such as RF, may be absent in early arthritis, and erosions may appear on plain radiographs only after a time lag of months to years. A very effective therapeutic option is administration of antagonists against the proinflammatory cytokine TNF-α. Meanwhile, long-term safety data demonstrate good tolerability profiles. However, the direct costs of treatment (10–20-fold higher than traditional DMARDs) are substantial.
As an investigator-initiated trial, HIT HARD was designed to be easy to conduct in a real life setting despite being a controlled study. Therefore, neither the MTX or ADA doses were adjusted and there was no stratification according to disease activity so that the ‘natural’ course of the disease on pre-defined treatment could be followed. Interestingly, most patients were treated within the potential window of opportunity of 3 months32 with a median disease duration of 1.8 months. These data also suggest that—if instituted very early—MTX treatment is a potent therapy, even though it is inferior in reducing radiologically visible damage. Of note, for practical reasons, a uniform MTX subcutaneous dose of 15 mg was used in all patients, and higher doses might have shown more favourable results, although the dose we administered is similar to the amount of bioavailable MTX used in most studies. The results of the first 24 weeks of treatment are in line with the OPTIMA study which used a very similar strategy but otherwise cannot easily be compared, since OPTIMA treatment after the initial 26 weeks was customised to five possible strategies according whether low disease activity was achieved at weeks 22 and 26.33 The safety profile in HIT HARD was good in both treatment arms with no major side effects and very good tolerability in this early patient population with limited co-morbidity. Thus, treatment decisions would not be influenced by the benefit–risk ratio.
As an investigator-initiated study, the trial had some limitations. There was a higher dropout rate in the MTX alone arm after 24 weeks; however, application of different statistical methods including predefined multiple imputations did not change the primary outcome (DAS28 at week 24) of a non-significant difference between both arms. Moreover, for regulatory purposes x-rays were performed as a standard of care. However, although imaging results were not obtained in all patients, there was still sufficient material to determine that radiological progression was higher in the MTX alone group. Funding was available only for the duration of the trial, and it would be very interesting to determine the future disease course in the two trial populations.
The results of the HIT HARD study raise some important questions. (1) Which patients benefited most regarding the prevention of radiographic progression? Since the numbers in the individual groups are small, this needs to be determined in larger trials, but initial evaluations suggest that patients with more erosive disease at baseline, a higher DAS28 score during treatment and ACPA positivity have more rapid progression. (2) Why are the radiographic and clinical responses different? This may be due to the direct and immediate influence of anti-TNF on osteoclast generation and differentiation, and to an anti-TNF effect on chondrocyte activation. (3) Which patients do show optimal outcomes after initial induction therapy and a subsequent switch to monotherapy? A few patients seemed to have lost response upon removal of ADA and so might have benefited from continued therapy.
Conclusion
In conclusion, the HIT HARD study provides some important information pertinent to treatment decisions. A (at least partial) disease reset may be within reach if RA is treated as early as possible, regardless of the strategy chosen, with a good safety profile. The strength of our study was the very short disease duration (1.6 or 1.8 months, respectively, in the two treatment groups). Combination therapy with MTX and a TNF inhibitor leads to rapid disease improvement which, however, is apparently masked by signs and symptoms in MTX monotherapy. One of the main questions in future treatment decisions will also be: is it economically justified to treat disease early with a costly, but immediately effective therapy which also reduces structural damage? Important answers here will be provided by future (bio)marker studies that identify those patients before treatment who will profit most from an induction approach.
Acknowledgments
The authors thank all patients who participated in the trial and the other members of the German HIT HARD study group: Hans Joachim Bergerhausen, Department of Rheumatology, Klinikum Duisburg; Arnold Bussmann, Rheumatology practice, Geilenkirchen; Winfried Demary, Rheumatology practice, Hildesheim, Andreas Engel, Rheumatology practice, Stuttgart; Andreas Kapelle, Rheumatology practice, Hoyerswerda; Andreas Krause, Department of Internal Medicine, Rheumatology and Clinical Immunology, Immanuel Krankenhaus Berlin; Ulf Müller-Ladner, Department of Rheumatology and Clinical Immunology, Kerckhoff Klinik, Bad Nauheim; Hans-Hartmut Peter, Department of Rheumatology and Clinical Immunology, Universitätsklinikum Freiburg; Andrea Rubbert-Roth, Department of Internal Medicine, Universitötsklinik Köln; Reinhold E. Schmidt, Department of Immunology and Rheumatology, Medizinische Hochschule Hannover; Matthias Schneider, Department of Endocrinology, Diabetology and Rheumatology, Universitätsklinikum Düsseldorf; Holger Schwenke, Rheumatology practice, Dresden; Joachim Sieper, Department of Internal Medicine, Rheumatology, Charité–Universitätsmedizin Berlin; Hans-Peter Tony, Department of Internal Medicine, Universitätsklinikum Würzburg; Jörg Wendler, Rheumatology practice, Erlangen.
We thank all study personnel and partners (DRFZ, KKS Charité) of the HIT HARD trial and the German Federal Ministry of Education and Research. The authors also thank Abbott GmbH & Co for supplying the trial medication adalimumab and placebo. We thank Dr S Kary and Professor A Zink for advice on trial design. We thank Professor Gromnica-Ihle, Professor Hein and Professor Gaubitz for their work as members of our data monitoring and safety board.
References
Supplementary materials
Web Only Data
Files in this Data Supplement:
Web Only Data
Files in this Data Supplement:
Web Only Data
Files in this Data Supplement:
Footnotes
-
Trial registration http://www.controlled-trials.com: ISRCTN36745608; EudraCT Number: 2006-003146-41.
-
Contributors All authors were responsible for study design, the collection, analysis and interpretation of all data, the writing of the article and the decision to publish.
-
Funding This study was funded by the German Federal Ministry of Education and Research (BMBF, grant number: 01KG0602), trial designation: High Induction Therapy with Anti-Rheumatic Drugs–HIT HARD. Adalimumab was provided by Abbott Co., Wiesbaden, Germany, under an unconditional scientific grant. Abbott had no influence on trial design, interpretation of the data or writing of the paper.
-
Competing interests GRB and KK have received honoraria from Abbott for speaking, board membership and lectures.
-
Ethics approval This study was conducted with the approval of the Berlin State ethics committee (Landesethikkommission Berlin).
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Correction notice This article has been corrected since it was published online first. The Acknowledgements section has been altered.