Article Text
Abstract
Aim To compare the extent of atherosclerosis in patients with psoriatic arthritis (PsA) and patients with cutaneous psoriasis without arthritis (PsC).
Methods In this cross-sectional study the authors compared patients with PsA with PsC patients. Psoriasis patients underwent a rheumatological assessment to exclude inflammatory arthritis. Ultrasonographic measurements of carotid total plaque area (TPA) and carotid intima-media thickness (cIMT) were performed. t Test was used to compare the imaging findings between the two groups. Multivariate linear regression analysis was used to assess the association between disease status and imaging findings after adjusting for potential confounders.
Results Overall, 125 PsA and 114 PsC patients were compared. There were no significant differences in age, gender or cardiovascular risk factors between the two groups. Patients with PsA exhibited greater TPA than did PsC patients (TPA (square root of area in mm2) 3.33±3.34 vs 2.43±2.72, p=0.03). This difference remained statistically significant in the multivariate regression analysis after adjusting for potential confounders (p=0.03). The difference in cIMT between the groups did not achieve statistical significance (p=0.09). The following disease-related variables were associated with increase in TPA in multivariate regression analysis among PsA patients: duration of PsA (p=0.04), highest Psoriasis Area and Severity Index recorded in the first 3 years of follow-up (p=0.02) and erythrocyte sedimentation rate (p=0.005).
Conclusions PsA patients suffer from more severe subclinical atherosclerosis compared with patients with PsC. This difference is independent of traditional cardiovascular risk factors and correlates with PsA disease duration, more severe skin disease and increased inflammatory markers.
Statistics from Altmetric.com
Several studies have reported an increase in incidence rates of cardiovascular morbidity among patients with psoriasis compared with the general population.1 ,2 The risk of having myocardial infarction in young men is 1.3 for mild psoriasis and 3.1 for severe psoriasis.2 Cardiovascular morbidity and mortality are also increased in psoriatic arthritis (PsA) patients. The risk of having myocardial infarction, ischaemic heart disease, hypertension, diabetes and dyslipidaemia was found to be increased in cross-sectional and longitudinal prospective cohorts.3 ,4
Carotid total plaque area (TPA) and carotid intima-media thickness (cIMT) can serve as surrogate measures for cardiovascular diseases (CVD). They can be used as prognostic indicators of vascular risk including coronary events, stroke and cardiovascular death in the general population and among patients with inflammatory arthritis.5–7 Several studies have shown that cIMT is higher in patients with psoriasis and PsA when compared with healthy controls.8–11 However, no study to date has compared the extent of subclinical atherosclerosis between PsA and psoriasis patients without arthritis. Since PsA is a systemic inflammatory disease that entails both skin and joint inflammation, we hypothesised that PsA patients are at a higher cardiovascular risk compared with psoriasis patients without arthritis. This hypothesis stems from a potential additive effect of chronic joint and skin inflammation that may lead to accelerated atherogenesis.
The present study aimed to compare the extent of ultrasonographic measures of atherosclerosis in patients with PsA and psoriasis without PsA (cutaneous psoriasis without arthritis (PsC)) and to determine whether PsA patients are at an increased cardiovascular risk compared with PsC patients.
Methods
Patients and setting
In this cross-sectional study patients with PsA were compared with those with PsC. Consecutive adult PsA patients who satisfied the CASPAR criteria12 were recruited from the University of Toronto Psoriatic Arthritis cohort that was established in 1978 as part of an ongoing prospective study. The patients are followed according to a standardised protocol every 6–12 months.13 The reference group of PsC patients were recruited from the University of Toronto Psoriasis Cohort. The cohort was described in detail previously.14 Briefly, all potential study subjects have a diagnosis of psoriasis confirmed by a dermatologist and have been assessed by a rheumatologist to exclude a diagnosis of PsA. The cohort was established with the aim of studying risk factors for the development of PsA. Patients are recruited mainly from dermatology clinics and phototherapy centres. All participants are followed according to the same protocol as in the PsA cohort and are assessed annually for symptoms or signs of arthritis. If inflammatory arthritis is diagnosed, the subject is considered to have developed the outcome of interest and is censored. This process ensures that all of the psoriasis cohort's subjects are free of arthritis. The study was approved by the University Health Network Research Ethics Board and all patients gave their informed consent.
Cardiovascular risk factor assessment
Blood pressure was measured and subjects were considered to have hypertension if they were taking antihypertensive agents or if they had a systolic blood pressure of >140 mm Hg and/or a diastolic pressure of >90 mm Hg. To determine whether patients were obese, height and weight were measured and body mass index was calculated. Blood samples were collected after 12 h overnight fasting and analysed for glucose, uric acid and lipid profile. Diabetes mellitus was considered to be present if glucose was >7 mmol/l or if antidiabetic medications were used. Patients were defined as smokers if they have ever smoked daily for more than 1 year. Current alcohol consumption was categorised as none, daily or social/occasional. Information about CVD events that occurred prior to enrolment was retrieved from the cohort's computerised database and was based on patients’ report and review of their medical records.
Assessment of disease activity and damage
Disease-related information included: age at diagnosis of psoriasis and PsA, current and ever use of non-steroidal anti-inflammatory drugs, disease modifying antirheumatic drugs and anti-TNFα agents. Tender, swollen and damaged joint counts were assessed on physical examination. An ‘actively inflamed’ joint was defined by the presence of stress pain and/or effusion in any of 68/66 joints. Clinically damaged joints (68 assessed joints) were defined as the presence of limitation of range of movement of >20% of the range, not related to the presence of joint effusion, presence of joint deformity, subluxation, loosening or ankylosis. Current psoriasis activity was determined by the Psoriasis Area and Severity Index (PASI).15 Severity of psoriasis was assessed by the highest PASI in the first 3 years of follow-up. Additional laboratory tests included erythrocyte sedimentation rate (ESR) and high-sensitivity C reactive protein.
Carotid ultrasound
A single trained physician (LE) performed all measurements following the study protocol. Scans were performed with a MyLab 30 (Esaote, Genoa, Italy) scanner with a linear LA523 7–13 MHz transducer (Esaote). The scan included detailed B-mode images of both right and left common carotid arteries as well as the carotid bulb, internal carotid and external carotid arteries. The mean cIMT was measured automatically at the far wall of the right and left common carotid arteries using an optimised ultrasound system for carotid imaging (QIMT tool; Esaote). Then the composite mean cIMT was calculated by averaging the common right and left cIMT values. The result was a single cIMT value per person, expressed in micrometres. An atherosclerotic plaque was defined as a localised thickening exceeding 1 mm as measured from the media-adventitia interface to the intima-lumen interface. The burden of atherosclerotic plaques was measured by the carotid TPA. This method correlates better with cardiovascular events than the cIMT.16 Each plaque was scanned in a longitudinal view until the maximum area of the plaque was in the plane of view. The image was then frozen and magnified and the plaque area was measured by tracing the perimeter with a cursor (figure 1). TPA was recorded as the sum of all plaques from the clavicle to the jaw in the right and left carotid arteries expressed in mm2. Reading of the ultrasound scans was performed independently from the scanning. The scans were recorded in digital media and read at a separate location and later date after blinding for personal data. Reproducibility and intraobserver variation were checked by rereading 25 images of the study subjects on different days. The intraobserver intraclass correlation coefficient for TPA was 0.94.
The image demonstrates ultrasonographic measurement of a plaque at the junction of the common carotid artery and the bulb in a longitudinal view. The cross-sectional area of the plaque is measured by tracing around the plaque with a cursor. The example in this figure has an area of 0.12 cm2.
Statistical analysis
Continuous data were described as mean and SDs and categorical variables as frequencies and percentages. Comparisons between the two categories were made using two tailed t tests for continuous variables and by the χ2 test for categorical variables. Since the distribution of the TPA was heavily skewed to the left, these data were transformed to square root of TPA. Pearson correlation coefficients were used to study the relationship between clinical variables and the imaging results. A multivariate linear regression model was used to estimate the correlation between imaging results and disease status (PsA vs PsC). Each of the ultrasonographic measures (cIMT or TPA) was separately taken as an outcome and the disease status was considered the key predictor. Adjustment was made for the following variables: age, sex, hypertension, diabetes mellitus, smoking, alcohol consumption, total cholesterol and triglyceride levels, use of lipid lowering agents and body mass index. Linear regression analysis was further used to determine the association between disease-related variables and TPA. Each initial model included only TPA as the outcome variable and a single disease-related factor as the explanatory variable, and then age and gender were added to the model as confounding variables. The effect of each factor was expressed as regression coefficients (β) along with their respective 95% CIs and reported with p values. The effect of covariates was considered as statistically significant if the p value from the two-sided Wald test was <0.05. The statistical computation was performed using SAS V.9.2.
Results
In all, 252 subjects were recruited and underwent ultrasound assessment; however, 13 subjects (three PsA, 10 PsC) were excluded from the analysis due to missing clinical or laboratory data. Overall, 125 PsA and 114 PsC patients were analysed. There were no significant differences between PsA and PsC in their mean age, gender or ethnicity; however, PsA patients had a longer duration of psoriasis (table 1). PsC patients exhibited more active psoriasis, reflected by their higher PASI scores; however, there was no difference between the groups in the highest PASI recorded. Most of the PsA patients had well-established disease with a mean duration of 14.4±10.8 years; only three patients had early PsA of less than 1 year duration. The two groups were comparable with respect to cardiovascular risk factors. Three PsA and four PsC patients had a positive history of a cardiovascular event, either MI or angina.
Demographics and clinical characteristics of the study population
Comparison of imaging findings between PsA and PsC
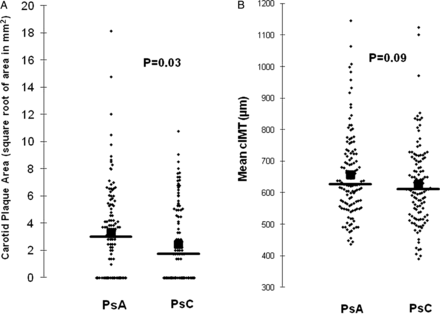
The prevalence of carotid plaques (TPA>0) was higher in PsA patients that however did not reach statistical significance (67% vs 56%, p=0.08). The mean number of carotid plaques was higher in PsA patients compared with PsC (1.9±2.1 vs 1.2±1.7, p=0.01). Furthermore, patients with PsA exhibited greater TPA than did PsC patients (TPA (square root of area in mm2) 3.33±3.34 vs 2.43±2.72, p=0.03) (figure 2A). Although the mean cIMT (µm) was numerically higher in PsA compared with PsC (655±135 vs 627±131, p=0.09), the difference did not achieve statistical significance (figure 2B).
Comparison of mean carotid total plaque area (A) and carotid intima-media thickness (cIMT) (B) between patients with PsA and PsC. Vertical line: mean; square sign: median. PsA, psoriatic arthritis; PsC, cutaneous psoriasis without arthritis.
The difference in TPA between PsA versus PsC remained statistically significant after adjusting for age, gender and cardiovascular risk factors (β=0.8, 95% CI 0.1 to 1.4, p=0.02). The β regression coefficients shown in table 2 represent the degree of change in the TPA (in square root of area in mm2) associated with PsA compared with PsC. This finding suggests that the higher TPA among PsA patients is not explained by differences in age and gender nor by excess in cardiovascular risk factors among PsA patients. These results were not appreciably changed after exclusion of the seven individuals who had previous cardiovascular events.
The association between TPA and PsA versus PsC by linear regression analysis
The correlation between disease-related variables and imaging findings
Since the difference in the extent of atherosclerosis between PsA versus PsC was not entirely explained by differences in the prevalence of traditional cardiovascular risk factors, we evaluated the correlation between disease-related factors and imaging findings. Variables that were relevant to PsA were assessed only among these patients; the remaining variables were assessed in the entire study population.
First, we assessed the Pearson correlation coefficients of disease-related variables with TPA and cIMT (table 3). A positive weak correlation was found for increasing TPA as the level of inflammatory markers increased. The Pearson correlation coefficient between TPA and ESR was 0.24 (p=0.009), while the correlation with high-sensitivity C reactive protein was of borderline significance (r=0.15, p=0.06). Furthermore, a positive correlation was found between ultrasonographic atherosclerosis and duration of PsA (TPA: r=0.35, <0.001, c-IMT: r=0.24, p=0.006) and duration of psoriasis (TPA: r=0.13, p=0.05, cIMT: r=0.2, p=0.002). Since age and gender have a strong effect on atherosclerosis progression the association between disease-related variables and TPA was further analysed by multivariable linear regression analysis (table 4). Both ESR and duration of PsA were significantly associated with TPA in the unadjusted as well as in the age- and sex-adjusted models. In addition, although the association between highest PASI and TPA was of borderline significance in the univariate model, it achieved statistical significance in the multivariate model suggesting that more severe skin disease is associated with higher plaque burden. The results were not appreciably changed after adjusting for traditional cardiovascular risk factor (data not shown).
Pearson correlation coefficients between disease-related variables and imaging findings
The association between disease-related variables and TPA among patients with PsA and PsC by linear regression analysis
Discussion
The present study demonstrates that patients with PsA have more severe subclinical atherosclerosis, as reflected by higher carotid TPA, compared with PsC patients. Traditional cardiovascular risk factors correlated with more severe carotid plaques among patients with both PsA and PsC; however, the more severe atherosclerotic disease among PsA patients was independent of these factors. Elevated ESR, longer PsA disease duration and the highest PASI score were associated with elevated TPA, suggesting that higher inflammatory burden, as reflected by these variables, may account for the more severe atherosclerotic changes in PsA compared with PsC.
CVD is the major cause of morbidity and mortality among patients with PsA and severe psoriasis.1–3 Furthermore, the prevalence of metabolic syndrome and its components is higher among patients with psoriatic disease compared with the general population and those with other types of articular disorders.9 ,17 ,18 ‘Psoriatic march’ was a term coined by Boehncke et al to describe the evolution of atherosclerosis in psoriatic disease.19 It suggests that the chronic systemic inflammation that is part of severe psoriasis and PsA leads to insulin resistance, resulting in endothelial dysfunction and atherosclerosis. Our findings support this hypothesis, as PsA patients suffered from more severe atherosclerotic disease compared with PsC patients, possibly due to higher systemic inflammatory burden due to the combination of skin and joint diseases. However, the effect of systemic inflammation on the progression of atherosclerosis was only partially mediated by traditional cardiovascular risk factors as the association between PsA and higher TPA was independent of these risk factors. This finding suggests that other unmeasured traditional and non-traditional risk factors or disease-specific risk factors account for the remaining excess risk.
Several studies in the past have assessed subclinical carotid atherosclerosis among patients with PsA. The main outcome measure in these studies was cIMT that was increased in PsA compared with the general population.11 ,20 ,21 We assessed two surrogate measures of subclinical atherosclerosis, cIMT and TPA. Although cIMT is more commonly used in research of cardiovascular risk, TPA that quantifies the extent of carotid plaques which represents more advanced atherosclerostic disease is likely a better surrogate measure for clinical cardiovascular events.22–24 Indeed, TPA was the only measure that differed between PsA and PsC patients. Previous studies in PsA have found that traditional cardiovascular risk factors were the main predictor for increased cIMT and plaques, and a few studies suggested that PASI and PsA disease duration are associated with more severe atherosclerosis; however, most studies did not find any association between disease-related factors and atherosclerosis.9 ,11 ,20 ,21 ,25 That lack of association may be explained by the combination of the small sample size of these studies and the strong effect that traditional cardiovascular risk factors have on the development of atherosclerosis. Furthermore, the cross-sectional nature of the studies does not allow measuring the cumulative effect of factors that fluctuate significantly over the course of the disease such as the activity of the skin and joint inflammation. In our study, we were able to find an association between TPA and several disease-related variables that supports the hypothesis that an increase in inflammatory burden is associated with worst outcome. Both the intensity of inflammation (level of ESR and highest PASI score) and the duration of exposure to inflammation (duration of PsA) were associated with worst atherosclerotic changes.
Our study was limited by several aspects. Its cross-sectional nature precluded a firm conclusion regarding any causal relation between disease-related predictors and atherosclerosis. Second, as our study population was recruited mostly from referral centres the generalisability of results may be limited to patients with the more severe spectrum of psoriatic disease. Another potential limitation is the lack of assessment of plaque characteristics which has been associated with vulnerability to rupture. Finally, we are aware that there are several potential cardiovascular risk factors that were not included in the analysis such as family history of CVD, physical activity and others. Lack of physical activity due to arthritis or joint damage may be a potential cause for the more severe atherosclerotic disease in PsA. It is possible that these factors may explain the difference in atherosclerotic burden between the two groups. Despite these limitations, the strengths of our study include its relatively large sample size, the use of two different quantitative measures to assess atherosclerosis and the accurate phenotyping of the study population. Furthermore, to our knowledge, this is the first study that directly compared the severity of atherosclerosis between PsA and PsC patients.
In conclusion, our study suggests that PsA patients suffer from more severe subclinical atherosclerosis compared with patients PsC. This difference is independent of traditional cardiovascular risk factors and correlates with markers of increased inflammation. Future studies should focus on identifying disease-related predictors for atherosclerotic progression among patients with psoriatic disease in order to elucidate the underlying mechanism of accelerated atherosclerosis in these patients.
References
Footnotes
Contributors All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors were likewise involved in the study conception and design, acquisition of data as well as analysis and interpretation of data. DDG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Funding The Psoriatic Arthritis Programme is funded in part by The Arthritis Society, Canadian Institutes of Health Research and the Krembil Foundation. The study was funded by a grant from Abbott Laboratories.
Competing interests None.
Patient consent Obtained.
Ethics approval Ethics approval provided by the University Health Network Research Ethics Board.
Provenance and peer review Not commissioned; externally peer reviewed.