Article Text
Abstract
Background The aetiology of rheumatoid arthritis (RA), a prototype immune-mediated inflammatory disorder, is poorly understood. It is currently unknown whether the disease process starts in the synovium, the primary target of RA, or at other sites in the body.
Objective To examine, in a prospective study, the presence of synovitis in people with an increased risk of developing RA.
Methods Thirteen people without evidence of arthritis, who were positive for IgM rheumatoid factor and/or anticitrullinated protein antibodies, were included in the study. To evaluate synovial inflammatory changes, all participants underwent dynamic contrast-enhanced MRI and arthroscopic synovial biopsy sampling of a knee joint at inclusion. Results were compared with knee MRI data and synovial biopsy data of 6 and 10 healthy controls, respectively.
Results MRI findings evaluated by measurement of maximal enhancement, rate of enhancement, synovial volume and enhancement shape curve distribution were similar between the autoantibody-positive subjects and the healthy controls. Consistent with these findings, all but one autoantibody-positive subject showed very low scores for phenotypic markers, adhesion molecules and vascularity, all in the same range as those in normal controls. The one person with higher scores had patellofemoral joint space narrowing.
Conclusion Subclinical inflammation of the synovium does not coincide with the appearance of serum autoantibodies during the pre-RA stage. Thus, systemic autoimmunity precedes the development of synovitis, suggesting that a ‘second hit’ is involved. This study supports the rationale for exploring preventive strategies aimed at interfering with the humoral immune response before synovial inflammation develops.
Statistics from Altmetric.com
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease primarily affecting synovial tissue in multiple joints. Despite increasing insight into the inflammatory pathways, and environmental and genetic factors that have a role in the initiation and progression of RA, the aetiology of the disease is poorly understood.
The synovium is the primary target of this disease. Systematic evaluation of the features of synovial inflammation in patients with early RA (signs and symptoms <1 year and the same if early RA is defined as disease duration <3 months) has shown that cell infiltration as well as the expression of cytokines, chemokines, granzymes, adhesion molecules and matrix metalloproteinases are, on average, similar to those observed in longstanding disease (>5 years' duration), when controlling for disease activity and use of antirheumatic drugs.1,–,4 Thus, early arthritis (as defined clinically) already represents chronic inflammation of the synovial tissue.5 This notion is supported by the observation that radiological damage can be seen in very early stages of the disease.6 Based on these findings we hypothesised that there might be a preclinical phase of RA, called pre-RA, characterised by inflammatory changes in the synovium before the onset of clinical signs and symptoms. This hypothesis is supported by the demonstration of synovial inflammation in clinically unaffected knee joints from patients with established RA.7 Furthermore, several animal models of RA have shown inflammatory synovial tissue changes in the latency phase of arthritis.7 Of note, however, the preclinical phase in the different animal models is relatively short, as arthritis develops within a few weeks after induction. Prospective data on the features of the synovium during the preclinical phase of RA are as yet not available, since it has been difficult in the past to identify individuals at risk of developing RA.8 Moreover, it is challenging to obtain synovial biopsy samples from people without arthritis.
Research has shown that IgM rheumatoid factor (IgM-RF) and anticitrullinated protein antibodies (ACPA) can be detected in the serum of patients with RA up to 14 years before the first clinical signs and symptoms of arthritis become manifest.9,–,11 These data show that immunological abnormalities precede the development of RA. In addition, detection of these autoantibodies allows us for the first time to identify individuals who are at risk of developing RA. We have recently shown that 20% of autoantibody-positive subjects with arthralgia developed clinical signs and symptoms of arthritis after a median follow-up of 28 months.12
To provide more insight into the question whether the disease process starts in the synovial tissue, we examined the synovium of IgM-RF- and/or ACPA-positive subjects without arthritis by dynamic contrast-enhanced (DCE) MRI, which is a sensitive tool for demonstrating synovial inflammation.13 14 In addition, we analysed arthroscopic synovial tissue samples from these same participants by immunohistochemistry, as histology is the ‘gold standard’ for evaluating synovial inflammation.
Patients and methods
Study participants
Thirteen IgM-RF- (serum level >12.5 kU/l; determined by IgM-RF ELISA Sanquin, Amsterdam, The Netherlands) and/or ACPA- (serum level >25 kAU/l; determined by anti-CCP2 ELISA Eurodiagnostica, Nijmegen, The Netherlands) positive subjects without arthritis (as determined by an experienced rheumatologist) were included. Twelve subjects with arthralgia were recruited from the clinical immunology and rheumatology outpatient clinic of the Academic Medical Center (AMC) in Amsterdam, of whom eight had been referred from the rheumatology department of the Jan van Breemen Institute, Amsterdam, and one was a first-degree relative of a patient with RA with arthralgia. Study participants were excluded if they had a history of arthritis, or if they had used disease-modifying antirheumatic drugs or corticosteroids for inflammatory joint complaints.
The control group for MRI consisted of six healthy people without any current or previous joint complaints and a normal knee joint at clinical evaluation. The control group for synovial biopsy comprised 10 subjects who underwent knee arthroscopy because of unexplained knee pain. None of these subjects showed inflammatory or degenerative joint pathology upon physical examination, arthroscopy or laboratory and radiological evaluation at inclusion or during 5 years of follow-up.15
Both autoantibody-positive individuals and controls gave written informed consent. This study was approved by the local medical ethical committee. The study was conducted according to the principles expressed in the ‘Declaration of Helsinki’.
Study design
At inclusion we collected demographics and disease activity parameters and carried out an x-ray examination of hands, feet and knee joints. Presence of the shared epitope was determined using sequence-based human leucocyte antigen-DRB1 typing. In addition, all autoantibody-positive subjects underwent DCE-MRI of the knee joint within 1 week before the arthroscopy. Follow-up consisted of yearly visits at which disease activity parameters were collected and x-ray examinations carried out. When a patient developed arthritis, an additional study visit was scheduled during which a second arthroscopy was performed and disease activity was assessed. Baseline MRI parameters and characterisation of the cell infiltrate and vascularity in the synovium were compared between the autoantibody-positive individuals and the control groups.
Disease activity parameters
At baseline we assessed disease activity by 68 tender and 66 swollen joint scores, patient's visual analogue scale for global disease activity (scale 0–100 mm), visual analogue scale for pain (scale 0–100 mm), erythrocyte sedimentation rate and serum levels of C-reactive protein. x-Ray examinations of hands, feet and the knee joint that was selected for arthroscopy were carried out to examine joint space narrowing and erosive changes.
MRI
MRI acquisition
Images were acquired on a 1.5 T MRI scanner (GE Signa Horizon Echospeed, LX9.0, General Electric Medical Systems, Milwaukee, Wisconsin, USA) using a 3D T1-weighted gradient echo dynamic sequence that consisted of 20 consecutive images of 20 slices with a temporal resolution of 22 s (TR/TE/flip 8·1/3·5/30, slice thickness 4 mm, field of view 18 cm, 256×256 matrix, axial orientation). The total imaging time was 7 min 19 s.
Autoantibody-positive individuals and healthy controls were placed supine with the knee joint centrally in the magnetic field in a dedicated extremity coil (quadrature detection). A 20-gauge needle infusion line was inserted in the right antecubital vein. Sixty seconds after the initiation of the dynamic protocol, a bolus of a Gd-DTPA contrast agent (0.1 mg/kg; Magnevist, Bayer Schering Pharma, Berlin, Germany) followed by a 15 ml saline chase was delivered at an injection rate of 5 ml/s using an automatic injection device (Spectris Solaris MR Injector; MEDRAD, Warrendale, Pennsylvania, USA).
MRI data analysis
Images were processed using an inhouse developed program running on MATLAB (MathWorks, Natick, Massachusetts, USA).16 This program analyses the time-dependent signal intensity changes (TIC) of every voxel in an imaged 3D volume. Of these TIC, maximal enhancement, maximal slope of increase and relative number of seven different TIC shape types were calculated, as described previously.14 Synovial volume was calculated as the number of enhancing voxels multiplied by the volume of each voxel.
Arthroscopic synovial tissue biopsy
All subjects underwent arthroscopic synovial tissue biopsy sampling of a knee joint.17 At least six synovial tissue biopsy specimens were collected for immunohistochemistry, as described earlier18 19 to minimise sampling error. The synovial biopsy samples were snap-frozen en bloc in Tissue-Tek OCT (Miles, Elkhart, Indiana, USA) immediately after collection. Cryostat sections (5 μm) were cut and mounted on Star Frost adhesive glass slides (Knittelgläser, Braunschweig, Germany). Sealed slides were stored at –80°C until use for immunohistochemistry.
Immunohistochemistry
Synovial tissue sections were stained using the following monoclonal antibodies: anti-CD3 (SK7; Becton Dickinson, San Jose, California, USA; T cells), anti-CD22 (CLB-B-ly/1,6B11; Sanquin, Amsterdam, The Netherlands; B cells), anti-CD55 (67; Serotec, Oxford, UK; fibroblast-like synoviocytes), anti-CD68 (EBM11; Dako, Glostrup, Denmark; macrophages), anti-CD138 (B-B4; Immunotech, Marseille, France; plasma cells), anti-von Willebrand factor (vWF; F8/86; Dako; blood vessels), anti-E-selectin (BBIGE4C5D11; R&D Systems, Minneapolis, Minnesota, USA), anti-ICAM-1 (MEM111; Sanbio, Uden, The Netherlands) and anti-VCAM-1 (IG11B1; Sanbio).
Staining of vWF was performed using a three-step immunoperoxidase method, as previously described.20 Staining of cellular markers was done on a Dako Autostainer universal staining system using a two-step indirect immunoperoxidase technique applying the Dakocytomation Envision System kit, and then counterstained with haematoxylin. For staining of adhesion molecules and growth factors, biotinylated tyramine was used for amplification, as previously described.21 As a negative control, irrelevant/isotype-matched immunoglobulins were applied to the sections instead of the primary antibody, or the primary antibody was omitted.
After staining of the slides the sections were analysed by semiquantitative analysis by two independent observers (MGvdS and GPMvdS, or MGvdS and TJS), as previously described.4 The expression of immunohistochemical markers was scored on a five-point scale (range 0–4). A score of 0 represented minimal expression, while a score of 4 represented high expression. For evaluation of the expression of CD68 cells, semiquantitative analysis was performed for intimal macrophages and synovial sublining macrophages separately. Minor differences between observers were resolved by mutual agreement.
Statistical analysis
Mann–Whitney U test was used to detect differences in MRI parameters between autoantibody-positive individuals and controls; χ2 test was used for the immunohistochemical analysis. A p value of <0.05 was considered significant. Values were expressed as median (range) or number. SPSS V.16.0 software (SPSS, Chicago, Illinois, USA) was used for the analysis.
Results
Study participants
Baseline characteristics of the autoantibody-positive individuals who were enrolled in this study are shown in table 1. Serum levels of IgM-RF and ACPA were elevated in eight and 10 of the autoantibody-positive individuals, respectively; five were positive for both autoantibodies. Titres of IgM-RF and ACPA in the positive subjects varied from 16 kU/l to 207 kU/l and from 35 kAU/l to 2591 kAU/l, respectively. Three patients had one shared epitope allele, one patient had two shared epitope alleles and the others were shared epitope-negative apart from one patient for whom no blood was available to determine shared epitope status. Although no clinical joint swelling was observed, most individuals had arthralgia of more than one joint. One person showed patellofemoral joint space narrowing of the knee joint, whereas all others had normal x-ray findings of hands, feet and the selected knee joint at inclusion. During follow-up of a median of 37 (range 25–45) months, four patients (31%) developed arthritis after a median period of 3 (1–6) months, consistent with our previous experience (after a median follow-up of 28 (19–39) months, 29/147 (20%) individuals with arthralgia developed arthritis).12 The presence of arthralgia at baseline was not related to the development of arthritis after follow-up. When arthritis was clinically manifest only one patient fulfilled American College of Rheumatology (ACR) 1987 RA criteria22 while three patients fulfilled the ACR/European League Against Rheumatism (EULAR) 2010 RA criteria.23 During follow-up two patients fulfilled the ACR 1987 RA criteria while all patients fulfilled the ACR/EULAR 2010 RA criteria.
Baseline characteristics of the autoantibody-positive subjects (n=13)
DCE-MRI reveals normal synovium in autoantibody-positive individuals who are at risk of developing RA
There were no clear-cut differences between autoantibody-positive individuals and controls in descriptive DCE-MRI parameters, TIC curve shape expression and synovial volume. All parameters at baseline were in the same range in both groups (table 2), even in those who developed arthritis over time (data not shown).
Dynamic contrast-enhanced TIC shapes in IgM-RF-positive and/or anti-ACPA-positive individuals without arthritis and healthy controls
Immunohistochemical analysis shows that the features of synovial biopsy samples are similar between autoantibody-positive individuals and controls
Of the 13 enrolled autoantibody-positive individuals, one was excluded from the synovial tissue analysis, because of insufficient quality of tissue sections according to the strict quality control system based on the absence of an intimal lining layer.
All but one autoantibody-positive individual showed low scores for T cells, B cells, plasma cells, macrophages, adhesion molecules and vWF, all similar to those observed in the controls (figure 1 and table 3). Scores for CD55 fibroblast-like synoviocytes were lower in autoantibody-positive individuals than in healthy individuals, which can presumably be explained by chance (table 3). The one autoantibody-positive subject with increased scores showed patellofemoral joint space narrowing on x-ray examination of the knee joint, but did not fulfil ACR criteria for knee osteoarthritis.24 Previous work has shown that the synovium is inflamed in patients with osteoarthritis.25 This patient did not develop arthritis during follow-up.
Synovial tissue expression of CD3 T cells, CD55 fibroblast-like synoviocytes and CD68 macrophages. (A) Autoantibody-positive individual. (B) Autoantibody-positive individuals with patellar-femoral joint space narrowing. (C) Healthy control (negative control). (D) Patient with active arthritis (positive control).
Semiquantitative scores (0–4) for phenotypic and vascular markers as well as adhesion molecules in synovial tissue of IgM-RF-positive and/or ACPA-positive individuals without arthritis and healthy controls
The immunohistochemical findings in the four patients who developed arthritis were in the same range as those in the other autoantibody-positive individuals, who did not develop arthritis during follow-up, as well as in the normal controls (data not shown).
Discussion
The aetiology of RA is still largely elusive, although significant progress has been made in understanding the pathogenetic mechanisms in established disease. Interestingly, work has shown that autoantibody formation may precede the development of clinical signs and symptoms of RA by several years.9 10 First, this observation provides important insights into the aetiological role of the humoral response in RA. Second, the measurement of RF and ACPA allows the identification of pre-RA patients before the development of arthritis,12 which was previously impossible.8 About one-quarter of IgM-RF- and/or ACPA-positive individuals with arthralgia develop arthritis after a median follow-up of 28 months.12
To determine whether the primary target of RA, the synovium, is already affected during the earliest phases preceding clinical signs and symptoms of RA, we performed MRI and synovial biopsy in IgM-RF- and/or ACPA-positive individuals without a history of arthritis who were prospectively followed up. The results presented here show for the first time that the synovium is not abnormal during this stage, even in those who develop arthritis during follow-up. Thus, systemic autoimmunity appears to precede the development of synovial inflammation in subjects who are at risk of developing RA.
A limitation of this study is the relatively small number of subjects evaluated. Obviously, the study design is challenging, as it is difficult to perform arthroscopy in subjects without arthritis strictly for research purposes. However, the results are strikingly consistent: we observed normal synovium by both MRI and immunohistochemistry in all subjects at baseline, including those who developed RA, except for the one patient who showed joint space narrowing on x-ray examination of the knee joint without osteoarthritis diagnosis. Moreover, in previous work we have been able to demonstrate significantly increased synovial inflammation in a study of comparable size, when comparing synovial biopsy samples from clinically unaffected joints of patients with established RA with those from normal controls.7
Another limitation is that we were able to perform arthroscopies from the knee joints only in this study. It is technically not possible to obtain sufficient synovial tissue for reliable analysis from clinically unaffected small joints. However, it appears unlikely that examination of synovial tissue samples obtained from other joints would have resulted in different findings, since (1) RA is a systemic disease and, accordingly, we have previously shown that there is a strong correlation between the features of paired synovial biopsy specimens from large and small joints in patients with RA,17 (2) six of the subjects in this study had arthralgia of the knee joint, (3) seven of the subjects also underwent ultrasound examination of the hand during routine patient care, which did not demonstrate synovitis of the small joints (data not shown) and (4) in a larger cohort it was recently shown that most autoantibody-positive individuals with arthralgia have normal results from an ultrasound examination of the small joints of the hands.26
It should also be noted that by prospectively selecting all subjects with an increased risk of developing RA (based on their autoantibody status and complaints of arthralgia), we have included those who might never develop clinically manifest arthritis. To date, it has not been possible to identify those people in whom systemic autoimmunity will proceed to evident RA in all cases. By applying this prospective study design and increasing participant numbers we hope to identify future biomarkers that can identify subjects who will go on to develop clinically manifest RA with an even higher likelihood.
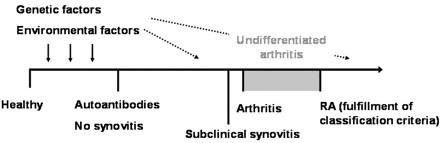
Genetic, stochastic and environmental factors may all have a role in the activation of the innate and adaptive immune system involved in the earliest, preclinical phase of RA, called pre-RA (figure 2). During this stage, which may last for several years, increased levels of C-reactive protein may be detected in the peripheral blood together with RF and/or ACPA.27 28 Our data suggest that the initial immune response leading to the production of autoantibodies takes place at sites other than the synovium. One candidate site is the lung, where various agents, including cigarette smoke, can trigger inflammation resulting in citrullination of specific peptides.29 Combined with a loss of tolerance to these specific citrullinated peptides, this may result in ACPA formation.29 30 Of note, Mahdi et al31 have shown that smoking is an independent risk factor for the development of ACPA-positive RA.
Timeline of autoantibody-positive rheumatoid arthritis (RA): the different stages of disease. Autoantibody formation may precede the development of clinical signs and symptoms of RA by several years. The presence of subclinical synovitis may probably last several weeks rather than months.
What determines the transition from the pre-RA phase to chronic synovitis? Obviously, this will be one of the major research questions for the near future.32 Possibly a ‘second hit’ is necessary to induce citrullination of proteins in the synovium. This might, for example, be a minor trauma or a viral infection. We previously found that citrullination may occur in the synovium in any form of arthritis.33 Conceivably, in the presence of pre-existing immunity against citrullinated antigens, this might lead to epitope spreading and autonomous progression of synovitis. ACPA immunoglobulin isotypes and epitopes recognised by ACPA have indeed been shown to evolve over time.11 34 35
Consistent with this hypothesis, ACPA reactivity against joint-specific epitopes has only been observed in patients with RA, but not in ACPA-positive relatives of patients with RA without arthritis.11
Based on studies in animal models of RA, presymptomatic synovitis may precede the development of clinical signs and symptoms of arthritis by several weeks.7 As we found in this study that the synovium was normal in subjects, who developed arthritis after a median follow-up of 3 months, we postulate that the phase of subclinical synovitis in RA is in the range of weeks rather than months (figure 2).
In conclusion, subclinical inflammation of the synovium does not coincide with the appearance of serum IgM-RF or ACPA antibodies during the pre-RA stage. Thus, systemic autoimmunity may precede the development of (subclinical) synovitis by several months to years and we therefore suggest that preventive strategies aimed at interfering with the humoral immune response before synovial inflammation develops should be explored. We hypothesise that in the presence of circulating ACPA, a ‘second hit’ may be required, leading to citrullination of peptides in the synovium, with subsequent broadening of the humoral response against citrullinated antigens in the joint and autonomous disease progression, as a result, in ACPA-positive RA.
Acknowledgments
The authors thank all study participants of the Pre-Synoviomics study who willingly participated in this study; I Burgman, C Lavini, G P M van de Sande, Dr T J Smeets and M Vinkenoog for their technical support and contribution to the study; Dr B M Lodde for editorial assistance; and the AMC arthroscopy team for arthroscopic synovial biopsy sampling.
References
Footnotes
-
Funding This research was funded by the Dutch Arthritis Association (nr 06-1-303) and the European Community's FP6 funding (AutoCure). This publication reflects only the authors' views; the European Community is not liable for any use that may be made of the information herein. The sponsors did not have any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the ethics committee of the Academic Medical Center – University of Amsterdam, Amsterdam, The Netherlands.
-
Provenance and peer review Not commissioned; externally peer reviewed.