Article Text
Abstract
Objectives During a multicentre study on juvenile idiopathic arthritis, wide variations were observed in bone shape, signal intensity and volume of joint fluid as shown by MRI which in part appeared to be unrelated to disease activity. A study was undertaken to examine these features in a cohort of healthy children.
Methods 88 children of mean age 9.8 years (range 5–15) underwent MRI imaging (T1-weighted Spin Echo and Spectral Selection Attenuated Inversion Recovery (SPAIR)) of the left wrist. The number of bony depressions, distribution and amount of joint fluid and the presence of bone marrow changes were assessed.
Results Bony depressions were present in all children, increasing with age from a mean of 4.0 in children aged 4–6 years to 9.2 in those aged 12–15 years (p<0.001)). 45 of 84 children (53.6%) had a high signal on SPAIR with a corresponding low signal on T1 in at least one bone. No associations were seen between bone marrow change (present or not) and sex (p=0.827) or sports club membership (p=0.616). All children had visible joint fluid in at least one of the joints assessed. No associations were seen between the presence of joint fluid and age group, except for the radius/scaphoid and capitate-scaphoid joints and a recess lateral to the hamate.
Conclusions It is important to be aware of the high prevalence of bony depressions, signal changes suggestive of bone marrow oedema and the volume of joint fluid seen in normal children. Such findings must be interpreted with care in children with suspected disease such as juvenile arthritis.
Statistics from Altmetric.com
Introduction
Ossification of the carpal bones starts at around 3 months of age and continues until skeletal maturation at 16–17 years.1 In most children, bone growth and modelling follow a specific sequential pattern with a constant ratio between carpal bone volumes.1 ,2 Radiographically, the small spherical ossification centres develop into multifaceted articulating bones with a well-defined cortex of varying thickness. With growth, the bony surface becomes slightly squared and irregular, typically seen along the radial aspect of the capitate—not to be mistaken for bony erosions or pathological change.1 The appearances of growing bones as assessed by MRI differ from x-rays. This is particularly noticeable with regard to bone shape as cortical bone returns a very low signal on all sequences and is therefore barely visible. Differences in imaging techniques as well as image artifacts such as chemical shift and movement artifacts may further distort the appearances. The diagnosis of bone erosions on MRI is therefore probably not so straightforward as was originally suggested in a recent uncontrolled study of 26 children with juvenile idiopathic arthritis (JIA), which defined a bone erosion as a sharply marginated bone lesion with correct juxta-articular localisation visible in two planes with a cortical breach seen in at least one plane.3
For adult patients with rheumatoid arthritis (RA), an MRI scoring system for disease activity of the wrists has been provided, including a reference atlas for the scoring of synovitis, bone marrow oedema and bone erosions according to the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) RA MRI scoring system.4 In this system, increased signal intensity (SI) on water-sensitive sequences with a corresponding low signal on T1-weighted images is considered diagnostic of bone oedema.5 ,6 In children, the hand skeleton undergoes developmental changes over time, such as enchondral growth and ossification, formation of bone (primary spongiosa) around the physis (growth plates) and conversion from haematopoietic to fatty marrow in neonates and infants. Bearing this in mind—and in order to construct a scoring system for wrist MRI in children with JIA—we aimed to establish normative standards for the growing wrist by gender, as assessed by MRI.
Methods
Healthy children aged 6–15 years residing in the city of Tromsø were invited to undergo an MRI of the left wrist. The invitation was announced on clip boards and via email at the University Hospital North Norway, Tromsø. During the period March to October 2009, 88 children accepted and were thus included.
On arrival at the radiology department the children were asked by the radiologist performing the scan whether they had sustained recent injuries to the left wrist. Children who had a recent injury were excluded.
The MR examinations were performed on a 1.5 T MR scanner (Philips Medical Systems, Best, The Netherlands), Intera model release 2.3 with master gradients and a four-element wrist coil. No sedation was used. A coronal T1 Fast Spin Echo (FSE), TR 561, TE 6.8, with three echoes and number of signal averages (NSA) was performed with 40 slices in three stacks. The slice thickness was 0.9 mm and acquired voxel size was 0.69×0.72×0.9 mm, with a reconstructed voxel size of 0.25×0.25×0.9 mm. Parallel imaging was used with a reduction factor of 1.6, giving a scan time of 4 min 11 s. The coronal T2 FSE scan, TR 3165, TE 70, 10 echoes, was fat suppressed using Spectral Selection Attenuated Inversion Recovery. The NSA was four, with 14 2.5 mm slices, giving an acquired voxel size of 0.31×0.40×2.5 mm and a reconstructed voxel size of 0.15×0.15×2.5 mm. Scan time was 3 min 56 s.
The MRI images were read by two paediatric radiologists (by consensus) using a high-resolution screen. The children were divided into four age groups: 6-7 years, 8-9 years, 10-11 years and 12-14 years. The following features were assessed: (1) number of bony depressions, as defined by a bony indentation other than the normal vascular channels on T1-weighted images, seen in the coronal plane and confirmed in at least one of the reformatted sagittal or axial planes; (2) distribution and volume of joint fluid (none, mild <2 mm or moderate ≥2 mm); and (3) the presence of high SI within the bone marrow on T2-weighted images with corresponding low SI on T1-weighted images for each of the carpal bones (yes/no): distal radius and ulna, scaphoid, lunate, trapezium, trapezoid, capitate, hamate, triquetrum and the bases of metacarpals 1–5.
Statistical analysis
A one-way between-group analysis of variance with age as the independent variable and number of bony depressions as the dependent variable was conducted to explore the impact of age on the number of bony depressions, with post hoc tests to determine where the differences among the age groups occurred. χ² Tests were used to examine possible associations between age and the proportion of children with bone marrow changes (dichotomised) and visible joint fluid (linear by linear associations with exact tests as appropriate). Based on total scores for bony depressions (subgrouped as 0–2, where 0 = <5 depressions, 1 = 5–10 depressions and 2 = 10 or more), bone marrow change and joint fluid, χ² tests were used to examine possible associations between sex, sports club membership, time of the year for the examination if appropriate (during March to 13 May and October or during 20 May to 30 September) and MRI findings. The analyses were performed using SPSS Version 17; p<0.05 was considered statistically significant.
Results
Eighty-eight healthy children (44 boys) with a mean age of 9.7 years (range 5–15) were examined. Seventy-nine (40 boys) were right-handed and the remaining 9 (6 boys) were left-handed. All were healthy active school children who participated in gymnastics at school (approximately 2 h weekly). Sixty-six (75%) of the children (36 boys) were members of at least one sports club, of which 40 (25 boys) did ball sports, 11 (2 boys) did dancing, ballet or gymnastics, 11 (5 boys) did alpine, swimming, riding or athletics while 4 boys attended martial arts classes.
Four of the T1-weighted sequences and four of the T2-weighted sequences were limited by artefacts and were excluded from further analysis. There were no differences according to sex for either of the imaging results, and the data were therefore pooled for analysis.
Bony depressions
The occurrence of bony depressions on the T1-weighted images is shown in table 1. There was a statistically significant difference in the total number of bony depressions between the four age groups, increasing with advancing age (F (3, 83)=14.7, p<0.001). The effect size, calculated using η2, was 0.4 (large effect). Post hoc comparisons using the Tukey HSD test indicated that the mean number of depressions differed significantly across the four age groups, except between 1 and 2, and 2 and 3. Figures 1–4 show typical appearances of carpal depressions for each of the four age groups. No differences in the total number of depressions were seen according to whether or not the child was a member of a sports club (p=0.528).
Examples of bony depressions (arrows) seen on the coronal T1 sequence in wrists of healthy children aged 6–7 years. (A) Irregular surface of the capitate and distal radius in a 7-year-old boy. (B) Bony depressions of the capitate in a 7-year-old boy. (C) Bony depression of the hamate in a 6-year-old girl.
Examples of bony depressions (arrows) seen on the coronal T1 sequence in wrists of healthy children aged 8–9 years. (A) Bony depression of the capitate in a 9-year-old boy. Note also the bony indentations of the proximal second and third metacarpals at the insertion of the ligaments (arrowheads). (B) Bony depressions of the pisiforme, capitate and hamate in an 8-year-old–girl. (C) Irregular surface and bony depression in the capitate, hamate and lunate in an 8-year-old boy.
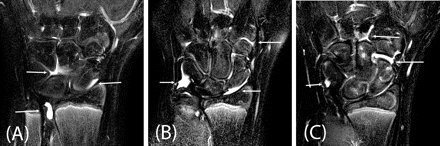
Examples of bony depressions (arrows) seen on the coronal T1 sequence in wrists of healthy children aged 10–11 years. (A) Bony depressions of the capitate and lunate in a 11-year-old boy. (B) Bony depressions in the capitate in a 11-year-old boy. (C) Bony depressions in the triquetrum in a 10-year-old girl.
Examples of bony depressions (arrows) seen on the coronal T1 sequence in wrists of healthy children aged 12–14 years. (A) Bony depression of the capitate in a 13-year-old girl. (B) Irregular surface of the capitate in a 14-year-old boy. (C) Bony depressions in the lunate in a 13-year-old boy.
Bony depressions in 84 healthy children (43 boys) as assessed by a T1-weighted Fast Spin Echo sequence
Joint fluid
All children had visible joint fluid in at least one of the joints assessed (table 2 and figure 5). No associations were seen between the presence of joint fluid and age group, except for the radius/scaphoid and capitate-scaphoid joints and a recess lateral to the hamate (table 2). Except for the second carpometacarpal joint which had a moderate volume of joint fluid in seven boys versus two girls (p=0.040), no associations were seen between the amount of joint fluid and sex. Similarly, no associations were found between the presence of visible joint fluid and sports club membership or time period.
Examples of normal intra-articular fluid in all four wrist compartments, radioulnar, radiocarpal, midcarpal and carpometacarpal joints in healthy children (arrows) shown on T2 Spectral Selection Attenuated Inversion Recovery (SPAIR) sequences in (A) an 8-year-old girl; (B) a 10-year-old girl; and (C) a 9-year-old boy.
Joint fluid (scored as yes or no) demonstrated in the wrist on T2-weighted SPAIR sequences in 84 healthy children
Medullary signal change
Forty-five (23 boys) of 84 children (53.6%) showed medullary signal changes indicative of oedema in at least one of the bones assessed, including the distal radius (table 3). No associations were seen between bone marrow change (present or not) and sex (p=0.827) or sports club membership (p=0.616). Twenty-seven of the 40 examinations performed during the snowy period versus 18 of 44 performed during summer revealed bone marrow changes in at least one of the assessed bones (p=0.017). Significant signal change within the distal radius was seen in two children, of whom one boy aged nine admitted to having had an extension trauma to the wrist while playing football a couple of days before the MRI examination (figure 6D). The remainder had no complaints or symptoms.
Examples of high signal on T2 Spectral Selection Attenuated Inversion Recovery (SPAIR) sequences with corresponding low signal on the T1 sequence suggestive of bone marrow oedema (arrows) in (A) the capitate of a 9-year-old boy; (B) the capitate and hamate of an 8-year-old girl; (C) the proximal four metacarpals in an 11-year-old boy; and (D) the distal radius in a 9-year-old boy who admitted to having had a mild extension trauma to the wrist 2 days earlier.
Bony signal changes (low signal on T1-weighted and high on T2-weighted SPAIR sequences) in 84 healthy children
Discussion
We have shown that, with growth, bony depressions can be seen in the normal developing hand skeleton and the number increases with age. The high prevalence of bone marrow signal suggestive of bone marrow oedema within the carpal bones as well as the volume of joint fluid are noteworthy, and should help to appropriately interpret future diagnostic tests.
The strengths of our study are the prospective design, the standardised state-of-the art MRI protocol used and the high numbers included. All children were healthy and without any symptoms or medication at the time of the examinations. All but two were Caucasian.
The high prevalence of non-specific heterogeneous marrow signal—that is, foci of low SI on T1 and high on water-sensitive images—was an unexpected finding and may represent bone marrow oedema, isolated foci of residual or reconverted haematopoietic marrow or a combination of these. Since all the children were healthy and since carpal bones are believed to develop in a pattern similar to that of the epiphysis of long tubular bones,7 the theory on residual red marrow is less likely. This is because epiphysial marrow conversion occurs within 6 months of the radiological appearance of the ossification centre8 and, as such, should be complete in the capitate and the hamate (two of the most commonly involved bones) by 1 year of age. Although the capitate was more often affected in the younger age groups, no age dependency was found for the other bones, inferring that the marrow changes do not represent a normal maturation process. The random distribution of changes with involvement of both distal and proximal bones supports this view as normal marrow conversion is reported to follow the same general distal to central sequence.9
Focal reconversion of fatty to haematopoietic marrow may represent another potential explanation of our findings. This is a physiological process mediated by the body's demand for red blood cells and tends to occur at times of physiological stress. It is therefore unlikely that reconversion of fatty to haematopoietic marrow would occur in a large proportion of healthy children as seen in this study.
Also, reconversion typically occurs in the opposite direction to physiological marrow maturation, beginning in the vertebrae and flat bones of the pelvis and extending to the appendicular skeleton.9 Although it may be patchy9 and asymmetrical,10 it seems unlikely that it would involve small foci within a single or just a few carpal bones without affecting the bones proximal to these.
This leaves us with focal bone marrow oedema as the most plausible explanation. This is supported by the prominent signal changes within the distal radius in a 9-year-old boy who initially did not report any recent trauma but subsequently, on direct questioning, admitted to having suffered a mild hyperextension injury while playing football. Although the findings of bone marrow change and the amount of joint fluid were unrelated to whether or not the child attended a specific sports club, this does not exclude mechanical stress as a possible cause, but rather suggests that self-reported sports activity is a poor marker for the true activity level in healthy physically active children.
La Hei et al studied the MRI finding of bone marrow oedema without fracture following trauma to the scaphoid bones in 41 patients. Patients were immobilised for 6 weeks and all improved or normalised after 3–6 months. However, the observational design did not allow any conclusions to be drawn on causality of the initial high bone marrow signal.12
The finding of an increasing number of carpal depressions with advancing age was not unexpected. Similar changes have been reported in an observational study of 31 healthy asymptomatic individuals aged 32–64 years who underwent wrist MRI (0.23T scanner, T1W 3D FFE). The authors diagnosed up to two small bone lesions resembling erosions in nearly half of the subjects. All lesions were small, the numbers increased with age and were most commonly located on the volar aspect of the lunate, scaphoid, trapezium/trapezoid, triquetrum and the capitate of the dominant hand.13 Except for the side preference which could not be accurately assessed in our study due to the small number of left-handed children, their findings support ours although the MRI machine and technique used differed between the two studies. The findings underscore the need for a baseline MRI in patients being followed for destructive bone disease.
All the children had fluid in at least one joint, of which approximately half had more than 2 mm in the first carpometacarpal and the trapezium/scaphoid joints. This amount has been judged to be pathological by others.4 One might speculate that the relatively large amount of fluid seen within these joints (ie, the radial aspects of the wrist) may reflect the relative use/importance of this part of the hand during daily activity. Furthermore, half of the children included in our study had fluid within the piso-triquetral recess. This contrasts with the findings of Barakat et al14 who, in a retrospective study of 35 patients aged 13–57 years, stated that fluid in this localisation may help in the diagnosis of reactive carpal synovitis.
In the present study we chose to obtain high resolution coronal T1-weighted images. With this sequence we obtained good quality images in the coronal plane with hardly any motion artifacts even in non-sedated children. One limitation of the study is that this sequence gives a relatively lower resolution in the reformatted axial and sagittal planes. However, the reformatted images were of sufficient quality to confirm the findings performed in the coronal plane.
In conclusion, the high prevalence of bony depressions, signal changes suggestive of bone marrow oedema and the amounts of joint fluid are significant and should therefore be accounted for in future scoring systems for disease.
Acknowledgments
The authors acknowledge Roy M Nilsen at the Department of Public Health and Primary Health Care, University of Bergen for statistical advice.
References
Footnotes
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the Regional Ethics Committee, North Norway (REK, Nord Norge).
-
Provenance and peer review Not commissioned; externally peer reviewed.