Article Text
Abstract
Objectives In a post-hoc analysis, we aimed to validate the Lupus Low Disease Activity State (LLDAS) definition as an endpoint in an systemic lupus erythematosus (SLE) Phase IIb randomised controlled trial (RCT) (MUSE [NCT01438489]) and then utilize LLDAS to discriminate between anifrolumab and placebo.
Methods Patients received intravenous placebo (n=102) or anifrolumab (300 mg, n=99; 1,000 mg, n=104) Q4W plus standard of care for 48 weeks. LLDAS attainment (SLE Disease Activity Index 2000 ≤4 without major organ activity, no new disease activity, Physician’s Global Assessment ≤1, prednisolone ≤7.5 mg/d and standard immunosuppressant dosage tolerance) was assessed. Associations with endpoints and LLDAS attainment differences between treatments were explored.
Results LLDAS attainment at Week 52 was associated with SLE Responder Index 4 (SRI[4]) and British Isles Lupus Assessment Group–based Composite Lupus Assessment (BICLA) (74/85[87%] and 62/84[74%] were also SRI[4] and BICLA responders, respectively; both nominal p<0.001). Only 74/159 (47%) of SRI(4) and 62/121 (51%) of BICLA responders reached LLDAS.
Anifrolumab-treated patients achieved earlier LLDAS, and more spent at least half their observed time in LLDAS (OR vs. placebo; 300 mg: 3.04, 95% CI 1.34 to 6.92, nominal p=0.008; 1,000 mg: 2.17, 95% CI 0.93 to 5.03, nominal p=0.072) vs placebo-treated patients. At Week 52, 17/102 (17%), 39/99 (39%) and 29/104 (28%) of patients on placebo, anifrolumab 300 and 1,000 mg, respectively, attained LLDAS (OR vs. placebo; 300 mg: 3.41, 95% CI 1.73 to 6.76, p<0.001; 1,000 mg: 2.03, 95% CI 1.01 to 4.07, nominal p=0.046).
Conclusions LLDAS attainment represents a clinically meaningful SLE outcome measure, and anifrolumab is associated with more patients who met LLDAS criteria versus placebo. These data support LLDAS as an SLE RCT endpoint.
Trial registration number NCT1438489; Post-results.
- anifrolumab
- systemic lupus erythematosus
- low disease activity state
- treat-to-target
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Attainment of low disease activity (LDA) is a standard of care in rheumatoid arthritis (RA), supported by empirical evidence of validity (i.e., association with improved long-term outcomes) and utility (discrimination of treatment response).1 2 In contrast, a well-defined LDA definition in systemic lupus erythematosus (SLE) was only recently identified as a key research goal.3 4 In response to this unmet need, increasing evidence suggests that the Lupus Low Disease Activity State (LLDAS) represents a clinically meaningful state with potential utility in both research and clinical settings.5
Patients with SLE who spend the majority of their time in LLDAS are protected from damage accrual, and LLDAS is also associated with better health-related quality of life (HRQOL) and is more stringent than expert opinion.5–8 Validation in a clinical trial setting is necessary to demonstrate the utility of LLDAS as a response measure in SLE randomised controlled trials (RCTs). Recently, rates of LLDAS attainment were demonstrated to differentiate treatments in a trial comparing azathioprine and mycophenolate in nonrenal SLE.9 Utility of a novel endpoint such as LLDAS in trials of novel therapies requires it to be attainable and to align with existing response measures, but also to offer additional information, and to allow for discrimination between active treatment and placebo. Here, we present a post-hoc analysis of a large Phase IIb SLE RCT dataset and demonstrate LLDAS utility.
Methods
MUSE trial design
LLDAS was evaluated in a post-hoc analysis of data from the 52-week MUSE RCT (NCT01438489) of anifrolumab in SLE.10 Patients (≥18–65 years old) with moderate to severe SLE were randomised 1:1:1 to receive intravenous placebo or anifrolumab 300 or 1,000 mg every 4 weeks for 48 weeks plus standard therapy. Patients met the American College of Rheumatology SLE classification criteria at screening, including positive antinuclear antibody ≥1:80 or elevated anti–double-stranded DNA (anti-dsDNA) or anti-Smith antibodies.11 Other inclusion criteria at screening were SLE Disease Activity Index 2000 (SLEDAI-2K) ≥6 (excluding points attributed to SLE headache or organic brain syndrome), ‘Clinical’ SLEDAI-2K≥4, a British Isles Lupus Assessment Group (BILAG) 2004 organ domain score of ≥1A or ≥2B and a Physician’s Global Assessment (PhGA; 0–3) score ≥1.0.12 13 Patients with active severe or unstable neuropsychiatric SLE or lupus nephritis were excluded. Randomisation stratification factors were SLEDAI-2K (<10 vs. ≥10), baseline oral corticosteroid (OCS) dosage (<10 vs. ≥10 mg/d prednisone-equivalent), and type I interferon (IFN) gene signature (IFNGS) based on a four-gene expression assay (test–high vs. test–low).10 A total of 305 patients received placebo (n=102) or anifrolumab (300 mg: n=99; 1,000 mg: n=104).
The MUSE primary endpoint was the difference from placebo in the percentage of responders at Week 24, defined as SLE Responder Index 4 (SRI[4]), with patients who withdrew or were unable to taper Day 85–Week 24 OCS dosage to <10 mg/d and ≤day 1 dosage considered to be nonresponders.14 Additional endpoints included BILAG-based Composite Lupus Assessment (BICLA), Major Clinical Response (MCR), BILAG flares (defined as either one or more new BILAG-2004 A items or two or more new BILAG-2004 B items compared with the previous visit) and patient-reported outcomes (PROs) including Lupus Quality of Life (LupusQOL) and Patient’s Global Assessment (PaGA).10 15 16 Nonresponse imputation of missing data was used for the binary outcomes and baseline-observation-carried-forward approach for continuous data following withdrawal from study or discontinuation of treatment, whereas intermittently missing data were imputed using the last-observation-carried-forward approach. The study was completed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. Written informed consent was obtained from all patients. Further details on MUSE design and endpoints have been published.10
Post-hoc validation of LLDAS as an outcome measure
LLDAS was conceptually defined as ‘a state which, if sustained, is associated with a low likelihood of adverse outcome, considering disease activity and medication safety’.5 Subsequently defined using consensus methodology, LLDAS is attained if all of the following items are met: (1) SLEDAI-2K ≤4, with no activity in major organ systems (renal, central nervous system, cardiopulmonary, vasculitis and fever) and no haemolytic anaemia or gastrointestinal activity; (2) no new features of lupus disease activity compared with the previous assessment; (3) PhGA (0–3) ≤1; (4) current prednisolone-equivalent dosage ≤7.5 mg/d; and (5) well-tolerated standard maintenance dosages of immunosuppressive drugs and approved biologics.
The published definition of LLDAS5 was applied post-hoc programmatically as a binary measure for each visit based on the collected and unblinded MUSE data. Details of the derivation of LLDAS are presented in the online supplement (online supplementary table S1). Results from statistical analyses are presented using point estimates, 95% CI where appropriate and nominal p-values. We first assessed the prevalence of LLDAS and then examined the association of LLDAS with SRI(4) responders with OCS taper at Week 24, and SRI(4), BICLA and MCR responders at Week 52. We then assessed the association between the number of flares throughout the study and LLDAS attainment at Week 52. Relationships between LLDAS attainment and PaGA scores and LupusQOL domains were explored. Details of the statistical methods used for these analyses are provided in the online supplementary appendix.
Supplementary file 1
Post-hoc application of LLDAS to discriminate between placebo and anifrolumab
A detailed description of the statistical methods and application of LLDAS to discriminate between placebo and anifrolumab treatment groups is provided in the online supplementary appendix. We compared the percentages of patients who attained LLDAS over time in placebo and anifrolumab treatment groups. We also compared the percentage of patients who spent more than 20%, 50% and 70% of their time in LLDAS, and who managed to sustain LLDAS across four, five, six or seven consecutive visits either during the whole study or after Week 12. Time to first LLDAS attainment also was compared between treatment groups. By using the approach recently described by van der Heijde et al,17 we generated heat maps of LLDAS and SRI(4) attainment across the entire study, sorted by treatment, SLEDAI-2K and IFNGS at screening.
Results
Patient characteristics
Key MUSE demographics and baseline characteristics are presented in the online supplementary table 2. A total of 305 patients with active SLE were enrolled, the majority of whom were anti-dsDNA–positive (Farr assay) and IFNGS test–high. Details have been published.10
MUSE efficacy endpoints
As reported, patients in both anifrolumab treatment arms were more likely to reach a range of prespecified endpoints compared with placebo.10 A greater percentage of patients receiving anifrolumab treatment achieved SRI(4) with OCS taper at Week 24, SRI(4) and BICLA at Week 52, and MCR (online supplementary figure S1).
Post-hoc validation of LLDAS as an outcome measure
To test the association of LLDAS with other measures, we first assessed LLDAS attainment, using data pooled from all treatment arms. LLDAS attainment was positively associated with, but more stringent than, standard endpoints. LLDAS was attained by 51 of 305 patients (16.7%) at Week 24 (figure 1A). At Week 24, 41 of 51 patients in LLDAS (80.4%) achieved the primary endpoint (SRI[4] with OCS taper; figure 1A). However, only 41 of 82 primary endpoint responders (50.0%) at Week 24 met the definition of LLDAS at the same time point (Cochran-Mantel-Haenszel (CMH) test χ2=68.06, p<0.001). Similar results were observed for a comparison of LLDAS with SRI(4) and BICLA at Week 52. At Week 52, 85 of 305 patients (27.9%) attained LLDAS, compared with 159 of 305 patients (52.1%) and 121 of 301 patients (40.2%) attaining SRI(4) or BICLA, respectively (figure 1B and C); 74 of 85 LLDAS responders (87.1%) were SRI(4) responders, but only 74 of 159 SRI(4) responders (46.5%) attained LLDAS (CMH χ2=49.20, p<0.001). Furthermore, 62 of 84 LLDAS responders (73.8%) met BICLA criteria, but only 62 of 121 BICLA responders (51.2%) attained LLDAS (CMH χ2=39.74, p<0.001). During the study, 44 of 305 patients (14.4%) met MCR criteria; of these, 29 of 44 (65.9%) also were in LLDAS at Week 52; correspondingly, 29 of 85 patients in LLDAS (34.1%) at Week 52 met MCR criteria (CMH χ2=25.62, p<0.001; figure 1D). Patients who attained LLDAS at Week 52 had a 75.2% lower BILAG flare rate during the study compared with those who did not attain LLDAS at the same time point. The annualised BILAG flare rate during the study for patients who met LLDAS criteria at Week 52 was estimated as 0.15 flares per patient-year (95% CI 0.08 to 0.27) compared with 0.61 (95% CI 0.45 to 0.83) for patients not meeting the LLDAS criteria (p<0.001).
Association of LLDAS with other endpoints for pooled patients with active SLE treated with placebo or anifrolumab. Percentages of patients meeting LLDAS (pink) and other endpoints (blue); (A) SRI(4) with OCS taper, at Week 24; (B) SRI(4) at Week 52; (C) BICLA at Week 52; (D) MCR. Nominal p-values were based on CMH test of independence, adjusting for treatment and randomisation stratification factors. Patients without BILAG A or B at baseline were excluded from the BICLA analysis. BICLA, British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment; CMH, Cochran-Mantel-Haenszel; LLDAS, Lupus Low Disease Activity State; MCR, Major Clinical Response; OCS, oral corticosteroid; SLE, systemic lupus erythematosus; SRI(4), SLE Responder Index 4.
LLDAS attainment was also associated with improved PROs. Patients who did or did not attain LLDAS at Week 52 had decreased PaGA from baseline of 23.0 and 9.1 mm on a 100-mm visual analogue scale, respectively (Wilcoxon signed rank test S=–1264 and S=–2,441, both p<0.001; figure 2A). At Week 52, patients in LLDAS had lower PaGA compared with patients not in LLDAS (F[1, 297]=38.93, p<0.001). Patients in LLDAS at Week 52 also had greater LupusQOL scores than did patients who did not attain LLDAS (figure 2B).
Association of LLDAS with PROs for pooled patients with active SLE treated with placebo or anifrolumab. (A) Mean PaGA scores at baseline and Week 52 by LLDAS attainment at Week 52. (B) Mean LupusQOL domain scores at Week 52 by LLDAS attainment at Week 52. The nominal p-values and delta for comparing the difference in mean scores between patients in LLDAS and those who did not attain LLDAS at Week 52 were based on an ANCOVA test adjusted for treatment, randomisation stratification factors and respective baseline domain scores. Nominal p-values for comparing baseline with Week 52 PaGA scores were based on a Wilcoxon signed rank test. ANCOVA, analysis of covariance; LLDAS, Lupus Low Disease Activity State; PaGA, Patient ’s Global Assessment; PROs, patient-reported outcome; QOL, Quality of Life.
Post-hoc application of LLDAS to discriminate between placebo and anifrolumab
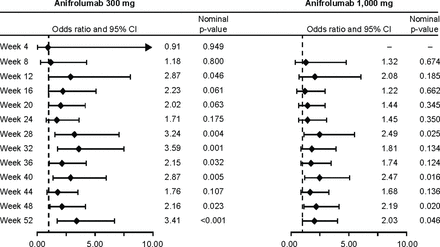
LLDAS criteria were met at least once by 36 of 102 (35.3%), 51 of 99 (51.5%) and 48 of 104 (46.2%) patients receiving placebo, anifrolumab 300 mg or anifrolumab 1,000 mg, respectively (OR vs. placebo; 300 mg: 1.97, 95% CI 1.08 to 3.58, p=0.027; 1000 mg: 1.63, 95% CI 0.90 to 2.95, p=0.103; table 1). Differentiation of LLDAS attainment in favour of anifrolumab over placebo was detected as early as Week 12 for anifrolumab 300 mg, with a range of ORs for subsequent visits from 1.71 at Week 24 (p=0.175) to 3.59 at Week 32 (p=0.001); this benefit of anifrolumab 300 mg was observed consistently after Week 24 with ORs >2 and CIs excluding 1 at all but one time point (figure 3). Differentiation was less pronounced for anifrolumab 1,000 mg, and was first detected at Week 28, with subsequent ORs ranging from 1.68 at Week 44 (p=0.136) to 2.49 at Week 28 (p=0.025). At Week 52, 17 of 102 (16.7%), 39 of 99 (39.4%) and 29 of 104 (27.9%) of patients on placebo, anifrolumab 300 mg and anifrolumab 1,000 mg, respectively, attained LLDAS (OR vs. placebo; 300 mg: 3.41, 95% CI 1.73 to 6.76, p<0.001; 1,000 mg: 2.03, 95% CI 1.01 to 4.07, p=0.046; figure 3). Anifrolumab-treated patients achieved LLDAS earlier than did placebo-treated patients (300 mg: χ2=6.39, p=0.012; 1,000 mg: χ2=2.44, 0.119; figure 4A). Patients receiving anifrolumab 300 or 1,000 mg spent greater total percentages of observed time in LLDAS than did patients receiving placebo (table 1). Patients receiving anifrolumab were also more likely to achieve LLDAS for longer periods of time (figure 4B and C). Greater percentages of anifrolumab-treated patients spent at least half of their observed time in LLDAS (OR vs. placebo; 300 mg: 3.04, 95% CI 1.34 to 6.92, p=0.008; 1,000 mg: 2.17, 95% CI 0.93 to 5.03, p=0.072; figure 4B). Furthermore, only 3 of 102 patients receiving placebo (2.9%) sustained LLDAS for seven consecutive visits, compared with 13 of 99 recipients of anifrolumab 300 mg (13.1%) and 11 of 104 patients receiving anifrolumab 1,000 mg (10.6%; figure 4C). Similar results were observed when analysis was restricted to the period after 12 weeks, when the onset of action of anifrolumab is assumed to have occurred (figure 4D).
Forest plot of LLDAS attainment comparing anifrolumab 300 mg (left) or 1,000 mg (right) at each time point during 52 weeks. ORs, 95% CIs and nominal p-values are based on a logistic regression model adjusted for randomisation stratification factors. LLDAS, Lupus Low Disease Activity State.
Time course of LLDAS attainment for patients with active SLE treated with placebo or anifrolumab. (A) Time to first attainment of LLDAS. (B) Percentages of patients attaining LLDAS for at least 20%, 50% and 70% of the observed period. (C) Percentages of patients sustaining LLDAS for at least 4, 5, 6 or 7 consecutive visits during the observed period. (D) Percentages of patients sustaining LLDAS for at least 4, 5, 6 or 7 consecutive visits during the period after Week 12. Nominal p-values were based on Grey’s test for each anifrolumab group versus placebo, or logistic regression models, adjusted for randomisation stratification factors. LLDAS, Lupus Low Disease Activity State.
Prevalence of LLDAS
We performed pro-forma power calculations to estimate sample sizes needed to detect differences to placebo in SRI(4) responders and LLDAS attainment at Week 52, assuming identical treatment effects to those observed in MUSE and this post-hoc analysis of MUSE data. Seventy-seven patients per group would be required for 80% power to detect a treatment effect for SRI(4) at a significance level of 5%. Fewer patients (n=61) per group would be necessary to achieve the same power for LLDAS attainment.
Graphical depiction of both attainment and retention of study endpoints across individual patients in clinical trials has recently been improved through the use of heat maps,17 and this approach may have particular utility in a relapsing-remitting disease such as SLE. Attainment and retention of LLDAS and SRI(4) across the duration of the trial, stratified according to screening SLEDAI-2K and IFNGS test status and treatment, are shown in figure 5. Both attainment and retention were numerically greater for SRI(4) than for LLDAS. LLDAS attainment occurred more often for anifrolumab-treated versus placebo-treated patients and was more frequent for patients with lower baseline disease activity. In placebo-treated patients, the likelihood of LLDAS attainment at Week 52 was lower for IFNGS test–high patients versus IFNGS test–low patients (at screening) (8/76 vs. 9/26, respectively; CMH χ2=4.19, p=0.041; figure 5).
Heat map of LLDAS and SRI(4) attainment. Responses according to LLDAS or SRI(4) over 52 weeks of study, stratified by treatment and baseline characteristics. LLDAS, Lupus Low Disease Activity State; IFNGS, type I interferon gene signature; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; SRI(4), SLE Responder Index.
Discussion
LLDAS was originally developed as a definition to use in treat-to-target pragmatic studies, and initial validation studies focused on the association of LLDAS with improved outcome in SLE.5 6 In RA, LDA is also used as a clinical trial endpoint, wherein the percentage of patients who attain LDA is used to compare treatments.2 Confirmation of the utility of LLDAS in SLE RCTs would provide a much-needed additional measure of treatment response. The potential for differences in rates of LLDAS attainment to permit discrimination between treatments is supported by the recently reported findings of Ordi-Ros et al 9 in their trial comparing mycophenolate and azathioprine in active nonrenal SLE; the study demonstrated that mycophenolate was superior to azathioprine in rates of LLDAS attainment. In our analysis, we provide novel evidence suggesting LLDAS utility as an endpoint in SLE RCTs. Partly because of the way LLDAS was defined, it was associated with existing response measures, including PROs, but was more stringent than other commonly used composite endpoints (SRI[4], BICLA), providing additional and complementary information. Our findings show that (1) LLDAS attainment and persistence were clearly differentiated between active treatment and placebo, indicating that the application of LLDAS can separate treatments and (2) anifrolumab treatment was associated with earlier, more frequent and more sustained LLDAS compared with placebo.
The definition of LLDAS was reached using a consensus methodology in response to the unmet need for such a measure, which was outlined in major reviews and by an international task force.3–5 Initial validation in a single-centre cohort demonstrated that a considerable percentage of patients attained LLDAS, distinguishing LLDAS from stringent definitions of remission, which are very seldom attained.18 Moreover, being in LLDAS for longer cumulative periods of time was associated with significant protection from damage accrual in two independent cohorts.5 6 Use of the operational definition of LLDAS was also recently found to be more stringent than expert opinion in assigning patients to LDA, and importantly, in a large prospective multinational study, that patients meeting the LLDAS definition had better HRQOL.7 8
As opposed to established trial endpoints such as SRI(4) and BICLA, which measure change from baseline, LLDAS represents a prespecified desirable outcome state.14 15 In analysis disregarding treatment, LLDAS attainment was associated with the MUSE primary endpoint of SRI(4) with OCS taper at Week 24, as well as with SRI(4) and BICLA at Week 52. However, although LLDAS was attainable, it was a more stringent endpoint—only approximately half of the patients who were SRI(4) or BICLA responders also met LLDAS criteria. This finding suggests that measuring LLDAS attainment provides additional information, complementary to that obtained using the previously established endpoints. Consistent with findings of a recent large multinational cohort study, LLDAS was associated with improvements in HRQOL compared with results for patients not achieving LLDAS, as measured by both the LupusQOL and PaGA measures.7 Together this suggests that in addition to change measures such as SRI(4) or BICLA, a stringent target state measure such as LLDAS has potential value in clinical trials in SLE.
Anifrolumab is a novel monoclonal antibody directed at the type I IFN receptor (IFNAR1) subunit, thereby blocking the actions of all IFN-α, IFN-β and IFN-ω cytokines.19 In the MUSE RCT, anifrolumab treatment was associated with greater percentages of patients who achieved the primary endpoint, SRI(4) with OCS taper at Week 24, as well as secondary endpoints, including SRI(4) and BICLA at Week 52, compared with placebo. Increasing the dosage from 300 to 1,000 mg did not lead to an increase in efficacy in MUSE. A greater rate of herpes zoster infection, as well as drop-out rate, in the anifrolumab 1,000 mg compared with the 300 mg group indicates a more favourable risk–benefit profile for the 300 mg dosage, which is the focus of the pivotal studies of anifrolumab.10 In the results presented here, an effect of anifrolumab on LLDAS was consistently seen across the different analyses, including greater percentages of patients attaining LLDAS at any time, as well as earlier and more sustained LLDAS attainment with more pronounced differentiation between anifrolumab 300 mg versus placebo. Our findings are consistent with the MUSE study results. These data suggest that LLDAS has utility to discriminate between treatment arms in an SLE RCT.
The SRI(4) endpoint was developed from an analysis of factors contributing to the ability to show the benefit of belimumab treatment versus placebo, and it has been used in several trials since.14 However, poor discrimination between active treatments and placebo is one of several issues that has plagued the evaluation of novel therapies for SLE, even when using endpoints derived from this measure and drugs addressing the same target.20 Clinically meaningful and more stringent endpoints could potentially allow for smaller trials, thereby permitting more agents to be studied. Also, though not intended to supplant measures of change such as SRI(4), endpoints that provide evidence of more pronounced therapeutic responses provide complementary information.
Illustration of drug trial outcomes by heat maps17 allows a unique oversight of overall patient outcomes over time, including the comparative time course of attainment and persistence of these outcomes. This method allows the comparison of endpoints, as well as the comparison of treatment arms. As provided in figure 5, LLDAS attainment was less frequent overall than SRI(4), consistent with its greater stringency as an endpoint, and not unexpectedly, was more often attained for patients with lower baseline disease activity. However, compared with placebo, treatment with anifrolumab was associated with increased LLDAS attainment and persistence overall, including patients with high baseline disease activity or IFNGS test–high status. Interestingly, for placebo-treated patients (receiving standard of care), LLDAS attainment was less likely for patients with a baseline IFNGS test–high score, suggesting that IFNGS status may be informative about patient outcomes receiving standard SLE therapy.
Limitations of this study include that it is a post-hoc analysis, although of prospectively acquired and adjudicated data. Additional studies of LLDAS utility in independent clinical trial datasets, and ultimately prospectively in RCTs, are needed to confirm our conclusions. A consensus also needs to emerge regarding operationalising LLDAS in clinical trials. For example, LLDAS is designed to be measured at a single point in time. Using a 30-day SLEDAI-2K,21 the disease activity domains refer to the preceding 30 days; fortunately, visit intervals in typical SLE clinical trials are 1 month. The assumption that gastrointestinal activity, which is not measured in the SLEDAI-2K or BILAG, is captured sufficiently in the PhGA also needs to be tested. A consensus on whether data on glucocorticoid and immunosuppressive drug treatment should be analysed similarly has not been reached. In the current study, several ways of handling these data were assessed, with little effect on the outcomes (data not shown), suggesting the pragmatic approach to recording treatment as of the day of assessment is sufficient.
In conclusion, we have evaluated the utility of LLDAS as an endpoint in a placebo-controlled randomised trial of a novel SLE therapy. The findings suggest that LLDAS is readily deployed in a trial setting, aligns with but is more stringent that existing measures of response thereby adding information complementary to these measures, is associated with HRQOL and is sensitive to detect an effect of an active treatment. The findings also suggest superiority of anifrolumab relative to placebo with respect to LLDAS attainment and persistence in patients with active SLE. The fact that LLDAS has been independently associated with improved long-term outcomes in SLE suggests the potential for clinically meaningful extrapolation of LLDAS attainment via the use of a novel therapy such as anifrolumab to the clinical context. Our findings support the inclusion of LLDAS as a measure of response in clinical trials of new treatments for SLE.
Acknowledgments
Editorial support was provided by Francis J Golder, BVSc, PhD, of Endpoint Medical Communications, Inc and Michael A Nissen, ELS, of AstraZeneca.
References
Footnotes
Handling editor Josef S Smolen
Contributors EFM: proposed the study design and wrote the manuscript. TT and AB: co-proposed the study design, undertook the statistical analysis and contributed to the manuscript. GI and RT: co-proposed the study design and oversaw the analysis and writing.
Funding AstraZeneca funded the MUSE study.
Competing interests EFM received research support, consultancy or honoraria from AstraZeneca, Bristol Myers Squibb, Glaxo Smith Kline, Janssen and UCB (Union Chimique Belge). TT, AB and RT are employees of AstraZeneca. GI was an employee of Medimmune during the conduct of this analysis; he is currently an employee of Regenxbio.
Ethics approval Independent Ethics Committee or independent Institutional Review Board approvals were obtained from all 101 clinical sites and all patients provided written informed consent in accordance with local requirements.
Provenance and peer review Not commissioned; externally peer reviewed.