Article Text
Abstract
Background Recently, disease activity states were developed for the Disease Activity index for PSoriatic Arthritis (DAPSA). Here, we assess if different DAPSA disease activity states are associated with different degrees of functional impairment and different extents of joint damage progression in patients with psoriatic arthritis (PsA).
Methods We used data from two pivotal trials of tumour necrosis factor (TNF) inhibitors in PsA (IMPACT II and GO-REVEAL) and identified patients in DAPSA remission (REM, ≤4), and low, moderate or high disease activity (LDA, ≤14; MDA, ≤28; HDA, >28) at 6 months. Across these groups we compared the functional scores (Health Assessment Questionnaire Disability Index, HAQ and physical component scale of the Short Form-36, PCS), and 1-year structural progression (PsA-modified Sharp/van der Heijde Score).
Results We identified 310 from GO-REVEAL and 130 from IMPACT II, with a mean (SD) baseline DAPSA of 48.8 (26.4) and 44.6 (17.9), respectively. HAQ scores increased across patients groups in the four DAPSA disease activity states, while PCS decreased (p<0.001 for both). The mean progression in the combined cohort was −0.47 for REM, −0.28 for LDA, −0.14 for MDA and 0.51 for HDA (p<0.001). This association was also significant in the individual trial cohorts, and in the subgroups of patients treated with TNF inhibitors or placebo. Higher DAPSA scores were significantly and independently associated with probability of structural progression in multiple analyses.
Conclusions Disease activity states of the PsA specific DAPSA score are highly valid for future use as endpoints in clinical trials or as targets in clinical practice.
Trial registration numbers IMPACT 2: NCT02152254; GO-REVEAL: NCT00265096.
- Psoriatic Arthritis
- Disease Activity
- Outcomes research
Statistics from Altmetric.com
Introduction
Active joint disease in psoriatic arthritis (PsA) may lead to cartilage and bone damage as well as impairment of physical function and quality of life.1 To quantify disease activity, a PsA specific disease activity score was recently developed and validated, the Disease Activity index for PSoriatic Arthritis (DAPSA).2 ,3 This index is a composite measure, which comprises swollen and tender joint counts, patient global and pain assessment and an acute phase reactant. Indeed, composite measures to evaluate activity of systemic arthritis have been shown to be more reliable than their individual components.3 ,4
Recently, also DAPSA disease activity states were established using clinical trial and observational data.5 However, like for any other disease activity instrument, in order to implement the DAPSA for use in clinical practice or clinical trials, or to serve as treatment target, for example, in the context of a treat-to-target strategy,6 evidence is needed to show the meaningfulness regarding important outcomes of the disease. Typically this covers two major domains, function and structure.7
In the present study, we aimed to provide evidence that DAPSA disease activity states are associated with different degrees of functional impairment and/or different extents of joint damage progression using data from two large clinical trials of PsA.
Methods
Study data
We analysed patient level data from two pivotal trials in PsA, the IMPACT II and the GO-REVEAL trial,8 ,9 which were kindly provided by the sponsor. In these RCTs, the effectiveness of the tumour necrosis factor (TNF) inhibitors infliximab or golimumab, respectively, had been investigated in patients with PsA who previously had not responded to conventional disease modifying antirheumatic drugs or non-steroidal anti-inflammatory drugs.
Analyses
At 6 months after the baseline visit, we identified patients in the different disease activity states according to the DAPSA, as previously defined: remission (REM, ≤4), low disease activity (LDA, ≤14), moderate disease activity (MDA, ≤28) and high disease activity (HDA, >28).5 The DAPSA is a numerical sum of the 66 swollen and 68 tender joint counts, the patient pain and global assessments (each on a 0–10 scale) and C-reactive protein (CRP) (in mg/dL), thus ranging from 0 to about 160 (ie, 154 plus CRP, with no natural upper limit for CRP).2
We first studied functional validity of the DAPSA states by assessing median scores of the Health Assessment Questionnaire Disability Index (HAQ), or of the physical component scale of the Short Form-36 (PCS, SF-36), across the different DAPSA states at 6 months. Since the HAQ was not assessed in IMPACT II, we limited this particular analysis only to the GO-REVEAL study. Since the SF-36 was measured in both trials, we present the data of each trial separately as well as pooled. Statistical comparisons were done using the non-parametric Jonckheere-Terpstra test for ordered groups. We also correlated DAPSA scores at 3 and 6 months with these two functional measures.
We then determined the rate of structural progression across these disease activity groups. We used the scorings obtained in the original trials, which employed a PsA-modified Sharp/van der Heijde (SvdH) scoring method. This method includes, in addition to the joints scored in rheumatoid arthritis (RA), the second through fifth distal interphalangeal joints of each hand, that is, leading to a scale that ranges from 0 to 528 (0–360 for the hands, 0–168 for the feet).10–12 In line with the analyses in the original trials, to quantify the structural progression over 1 year, we present the means across the four groups because overall progression was low and medians of radiographic progression are expected to be 0, and thus not informative. Statistically, however, due to non-normal distribution of these progression data, we used the Jonckheere-Terpstra test for comparison. In addition, we performed logistic regression analysis adjusting for baseline structural damage and type of treatment (TNF-inhibition or control). We modelled the association of DAPSA with 1-year structural progression using the 6 months measurement, the time-integrated DAPSA from 0 to 6 months and the time-integrated DAPSA from 0 to 12 months in three different analyses.
Results
We identified 310 patients from GO-REVEAL and 130 from IMPACT II, with a mean (SD) baseline DAPSA of 48.8 (26.4) and 44.6 (17.9), respectively (table 1). For additional characteristics, such as skin involvement and other manifestations, we refer to the original publications of the two trials.8 ,9
Patient characteristics at baseline
When we assessed functional validity, we observed highly significant differences across the four disease activity states, whether using HAQ scores (GO-REVEAL) or the SF-36 PCS (GO-REVEAL, IMPACT II and trials combined) with p<0.001 for all analyses (Jonckheere–Terpstra test; figure 1). Also, the DAPSA scores at 3 and 6 months correlated significantly with the respective HAQ sores (0.67 and 0.65, respectively) and the SF-36 PCS scores (0.53 and 0.65, respectively; see online supplementary table S1).
Functional validity. Box plots showing the functional levels of patients in the GO-REVEAL trial (A and B), the IMPACT II trial (C) and the combined trial population (D), for the PCS of SF-36 (A, C and D) or the HAQ (B). The differences across REM, LDA, MDA and HDA were statistically highly significant (p<0.0001) in each of the four analyses (tested by Jonckheere-Terpstra test for non-parametric comparisons of multiple ordinal groups). DAPSA, Disease Activity index for PSoriatic Arthritis; HAQ, Health Assessment Questionnaire Disability Index; HDA, high disease activity; LDA, low disease activity; MDA, moderate disease activity; PCS, physical component score; REM, remission; SF-36, Short Form-36.
Supplemental material
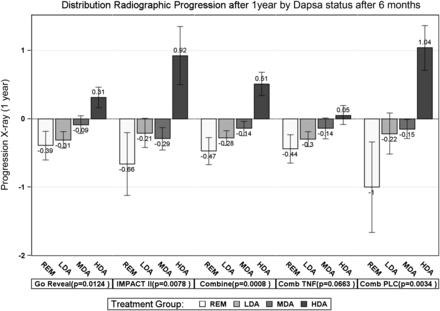
Next we investigated the validity of DAPSA states in regards to radiographic progression. Overall there was only very little progression in the two trials, with 18.9%, 10.9% and 6.1% of patients progressing by ≤0.5, ≤1 or ≤2 points on the SvdH scale, respectively, over 1 year. The mean overall progression was very low, namely 0.02 (SD: 1.68) per year; the median was 0 (Q1/Q3: −0.25/0.25), in fact, the vast majority of patients showed no progression, as can be determined from inspection of the respective probability plots (see online supplementary figure S1). Stratified by regimen, mean progression was −0.18 (1.24), and median progression was 0 (−0.50; 0.25) for all TNF-inhibitor-treated patients; for placebo-treated patients the mean was 0.42 (2.25) and the median was again 0 (−0.25; 0.25). The progression of joint damage was significantly different across the disease activity states (Jonckheere–Terpstra test, see figure 2). This was especially seen for the control arms (p=0.003), and also for the total trial populations; in line with their effects on structural progression, the damage changes across disease activity states in patients receiving TNF inhibitors only showed a trend (p=0.066).
Structural validity. Mean progression (including SEs) of structural damage according to the total Psoriatic arthritis adapted Sharp/van der Heijde Score, for patients in REM, LDA, MDA and HDA. Five different analyses are presented, from left to right: GO-REVEAL only (statistical comparison across the four disease activity groups by Jonckheere-Terpstra test: p=0.012), IMPACT II only (p=0.008), the combined trial population (p<0.001), the combined TNF-inhibitor-treated patients from both trials (p=0.066) and the combined placebo (methotrexate)-treated patients from both trials (p=0.003). DAPSA, Disease Activity index for PSoriatic Arthritis; HDA, high disease activity; LDA, low disease activity; MDA, moderate disease activity; REM, remission; TNF, tumour necrosis factor.
Finally, logistic regression analysis showed a significant association for DAPSA values at 6 months with structural progression from 0 to 12 months, which remained significant if we adjusted for baseline radiographic score and the type of treatment received (TNF-inhibitor vs control). We also used the time-integrated DAPSA score from 0 to 6 months, or from 0 to 12 months instead of the 6 months DAPSA in the model which was also significant for the three different endpoints of progression (see online supplementary table S2).
Discussion
The DAPSA is employed for the assessment of disease activity in patients with PsA. While it is sensitive to change using clinical trial and observational data and can be used to determine the traditional disease activity states including REM, it is also highly relevant to gather evidence regarding the validity of these states with regard to important outcomes that are linked to disease activity.13 The traditional outcomes in PsA are disability and joint damage. Here, we show that patients in the different disease activity states have different degrees of functional impairment, increasing almost linearly from REM to HDA; indeed, the HAQ disability score is twice as high in patients with HDA than in those with LDA, who essentially have a normal HAQ (∼0.25).
Importantly, also with respect to joint damage the DAPSA was significantly correlated with progression of structural changes, since the probability of progression increased with increasing DAPSA states.
DAPSA constitutes an instrument developed primarily to reflect the arthritis aspects of PsA. Several other instruments, such as Psoriatic Arthritis Disease Activity Score and Composite Psoriatic Disease Activity Index capture additional important variables related to psoriatic disease, such as physical function, skin and nail involvement or enthesitis. It is currently still debated if a PsA instrument should comprise only the arthritis activity, and other validated measures should be used to determine other manifestations of the disease, as needed, or to encompass the totality of the disease.14 DAPSA does not require transformation nor a calculator and the joint count used is related to PsA and not to RA, contrasting measures often used in clinical trials which only include 28 joint counts.
The focus on clinical trial data may constitute a limitation of this study. However, to our dismay we do not have radiographic scores available in our observational PsA cohort. Moreover, clinical trials include data from many centres and thus may be seen as reflecting PsA more broadly than patients coming from a single centre. As a strength of our study, the data from both clinical trials are confirmatory and the functional data have been cross-validated by using the HAQ as well as the PCS of the SF-36. Of particular note, the significant structural data in relation to DAPSA were detected despite the low progression of damage encountered in PsA trials.
In summary, in addition to the previously revealed evidence that DAPSA exhibits content, discriminant and face validity as well as sensitivity to change, it has now been shown that the disease activity states defined for the index are clearly corresponding to functional levels and structural changes, Thus, the DAPSA shows solid validity, regarding two of the most important outcomes of PsA, namely disability and damage.
Acknowledgments
The authors thank Dr Daniel Baker and his team from Janssen for the kind provision of the clinical trial data.
References
Footnotes
Handling editor Tore K Kvien
Contributors DA and JSS: design, analyses and write-up. FA: analysis.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.