Article Text
Abstract
Objective: To assess, firstly, the validity of the enthesis index published by Mander (Mander enthesis index (MEI)) and, secondly, to investigate whether it is possible to define a new enthesis index that is less time consuming to perform with at least similar or better properties.
Methods: Data from the OASIS cohort, an international, longitudinal, observational study on outcome in ankylosing spondylitis, were used. In this study, measures of disease activity, including the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the MEI, were assessed regularly in 217 patients. With the MEI, for each measurement period independently, a process of data reduction was performed to identify the entheses most commonly reported as painful by the patients. A more concise enthesis index was constructed with aid of the entheses found in this way. Correlations with measures of disease activity were used to test the validity of several entheses indices.
Results: Reduction of the number of entheses from 66 to 13 and omitting grading of the intensity of pain resulted in an index which was named the “Maastricht Ankylosing Spondylitis Enthesitis Score” (MASES). The MASES (range 0–13) has much greater feasibility than the MEI (range 0–90). However, up to 21% of patients with a score >0 on the MEI were not identified by a score on the MASES >0. Only 2.1% of the patients with an original enthesis score >0 had an original score on the MEI >3 (range 0–90) and it can be questioned whether a low score on the MEI index represents clinically important enthesitis. The Spearman correlation coefficient between the MASES score and the MEI was 0.90 and between the MASES and the BASDAI was 0.53 compared with a correlation of 0.59 between the MEI and the BASDAI.
Conclusions: MASES seems to be a good alternative to the MEI with much better feasibility.
- ankylosing spondylitis
- outcome
- enthesitis
- AS, ankylosing spondylitis
- ASAS, ASsessment in Ankylosing Spondylitis
- BASDAI, Bath Ankylosing Spondylitis Disease Activity Index
- CRP, C reactive protein
- ESR, erythrocyte sedimentation rate
- MASES, Maastricht Ankylosing Spondylitis Entheses Score
- MEI, Mander enthesis index
- MRI, magnetic resonance imaging
- NSAIDs, non-steroidal anti-inflammatory drugs
- OMERACT, Outcome Measures in Rheumatoid Arthritis Clinical Trials
- VAS, visual analogue scale
Statistics from Altmetric.com
- AS, ankylosing spondylitis
- ASAS, ASsessment in Ankylosing Spondylitis
- BASDAI, Bath Ankylosing Spondylitis Disease Activity Index
- CRP, C reactive protein
- ESR, erythrocyte sedimentation rate
- MASES, Maastricht Ankylosing Spondylitis Entheses Score
- MEI, Mander enthesis index
- MRI, magnetic resonance imaging
- NSAIDs, non-steroidal anti-inflammatory drugs
- OMERACT, Outcome Measures in Rheumatoid Arthritis Clinical Trials
- VAS, visual analogue scale
In 1995, the “ASsessment in Ankylosing Spondylitis” (ASAS) working group was formed in order to select a core set for outcome assessment in ankylosing spondylitis (AS).1 This international working group consists of clinical experts, clinical epidemiologists, representatives of the pharmaceutical industry, and representatives of patient leagues who share their expertise in the field of AS. In 1998, a definite core set was selected, to be used in various clinical settings, which consisted of different domains with specific instruments for each domain.2 For example, the domains selected to evaluate disease controlling treatment are function, pain, spinal mobility, patient’s global, stiffness, peripheral joints and entheses, acute phase reactants, spine and hip radiology, and fatigue. For all domains except entheses and fatigue, a specific instrument was selected.
Enthesitis is a primary clinical feature in AS. In 1987, Mander et al published an instrument to investigate enthesitis in AS (the Mander enthesis index (MEI)).3 A total of 66 entheses are investigated by local pressure, and intensity of pain is graded on a 0–3 scale (0=no pain; 1=mild tenderness; 2=moderate tenderness; 3=wince or withdraw). This instrument, however, is neither widely used in daily practice nor in clinical trials. The members of the ASAS working group questioned the feasibility of the MEI. Applying the MEI is time consuming; there may be a potential discrepancy between the reaction of the patient and its interpretation by the doctor; and applying pressure on all sites in active enthesitis may be unacceptable to the patient. Therefore the ASAS working group suggested that more research should be carried out in this field before selecting an official instrument.
To assess the appropriateness of an instrument to measure outcome, all aspects of validity need to be examined. To standardise the nomenclature of validity, the OMERACT (Outcome Measures in Rheumatoid Arthritis Clinical Trials) filter has been proposed.4 The three domains of the OMERACT filter are truth (validity), discrimination (reproducibility and responsiveness), and feasibility.
To be able to relate the level of enthesitis with the overall level of disease activity, an instrument to assess disease activity should be selected. However, in AS, currently no “gold standard” exists for measuring disease activity. Objective measures such as C reactive protein (CRP) and Westergren erythrocyte sedimentation rate (ESR) correlate poorly with clinical disease activity.5 A self administered questionnaire has therefore been developed that better reflects clinical disease activity in AS: the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). This instrument has been shown to be valid, reproducible, and responsive to change.6–8 Also widely used to measure disease activity is a 10 cm visual analogue scale (VAS) to be completed by the patient and by the doctor separately.
This study aimed, firstly, at assessing the validity of the MEI and, secondly, at investigating whether it is possible to modify the MEI to produce a less time consuming index with at least similar validity.
PATIENTS AND METHODS
Patients
For this study we used data from the OASIS cohort; an international, longitudinal, observational study on outcome in AS with follow up visits according to a fixed protocol. Data from this cohort have been previously reported.5 Consecutive outpatients with an established diagnosis of AS according to the modified New York criteria were included in 1996. We here will report data of patients from the University Hospital Maastricht, and the Maasland Hospital Sittard, the Netherlands and the University Hospital Gent, Belgium, all secondary and tertiary referral centres. Data from baseline, one year follow up, and two year follow up will be presented. At these visits patients completed a number of questionnaires and underwent a clinical examination including the MEI.
Mander enthesis index
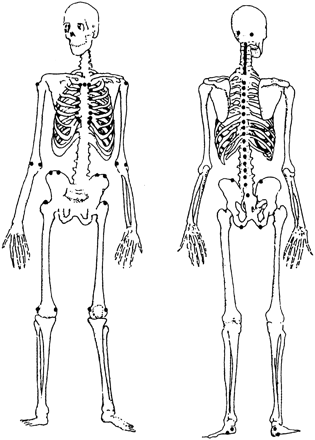
To assess the MEI, the investigator applies pressure over 66 different entheses accessible to palpation. The patients’ response to firm palpation over these entheses is noted (0=no pain; 1=mild tenderness; 2=moderate tenderness; 3=wince or withdraw). The following sites are included in the index: the nuchal crests, the manubriosternal joint, the costochondral joints, the greater tuberosity and the medial and lateral epicondyles of the humerus, the iliac crests, the anterior superior iliac spines, the greater trochanter of the femur, the medial and lateral condyles of the femur, the insertion of the Achilles tendons and plantar fascia to the calcaneus, the cervical, thoracic, and lumbar spinous processes, the ischial tuberosities, and the posterior superior iliac spines. Figure 1 shows the entheses included in the MEI. Some of the sites are scored individually whereas others are scored as a group, with the highest scoring site being recorded for the group as a whole. The sites that were grouped were the nuchal crests, the costochondral joints, the cervical, thoracic, and lumbar spinous processes. After grouping, a maximum score of 90 can be achieved.
Mander enthesis index. Reproduced with permission of the authors and the copyright holders from reference 3. Copyright @ 1987 Annals of the Rheumatic Disease.
Measures of disease activity
The BASDAI consists of six questions on fatigue, pain of the spine and hips, pain or swelling of the peripheral joints, localised tenderness as a proxy for enthesitis, and severity and duration of morning stiffness. The questions are answered on a 10 cm VAS, anchored with the labels “none” and “very severe” at either end of the first five questions, and with ”0 hours” and “two hours” at either end of the question on duration of morning stiffness. The mean of the two scores for morning stiffness counts as one variable. The final score is defined by calculating the mean of the five items. Scores range from 0 (best) to 10 cm (worst). When values were missing, at most one out of six questions from the BASDAI was substituted by the patient’s mean. In the case of a missing value for questions 5 or 6, both dealing with morning stiffness, the remaining question counted as the mean of questions 5 and 6.
A single item 10 cm VAS, concerning the degree of disease activity, and to be completed by both the patient and the doctor independently was anchored “no disease activity” at 0 cm and “very severe activity” at 10 cm.
The ESR was assessed by the Westergren method (mm/1st h; normal range for men 0–7, for women 0–12) and CRP by the turbidimetric method (mg/l; normal range 2–9). The lowest detection limit for CRP was 2 mg/l and patients with undetectable levels were assigned 0.
Statistical analysis
Data at baseline, one and two years’ follow up were analysed independently. Initially, the doctor recorded all 66 different entheses included in the MEI without grouping. After grouping, an enthesis score according to Mander was calculated for every patient. As a next stage, for each enthesis the original gradation of pain on the MEI was recoded dichotomously into “no pain” or “painful”. An original score of 0 was regarded as no pain; original scores ranging from 1 to 3 were regarded as painful. Only data from patients with a score on the MEI of >0 were used for developing modifications of the MEI.
In the following analyses a process of data reduction was performed. Baseline, one year follow up, and two year follow up were analysed independently. Frequency tables were used to determine which specific enthesis was scored painful most frequently at the specific time. This enthesis was noted and all patients reporting this enthesis as painful were not taken into consideration in the subsequent step. In the remaining patients, a similar analysis with use of frequency tables was performed to determine which enthesis was scored most frequently as painful. Similarly, all patients reporting this particular enthesis as painful were not included in subsequent steps. This process was repeated until up to 80% (arbitrarily chosen) of all patients with a score on the MEI >0 were included. If an equal amount of patients reported two different entheses as most frequently painful at the same analysis, we selected the point that was anatomically the most easy to localise. The entheses detected in this way at baseline, one year, and two years were taken together. If an enthesis was situated on the right or left side of the body, the contralateral enthesis was also included. Because we wanted an index as concise as possible, we decided, additionally, to decrease the number of entheses by omitting entheses more difficult to localise and entheses neighbouring those already selected.
As a last step the MEI, and the two modified MEIs were calculated with and without gradation of the intensity of pain.
Braun et al recently used an enthesis index in a study on infliximab in AS, composed of 12 entheses which are reported to be commonly affected in the inflammatory process in AS (major entheses index).9–11 This major entheses index includes the iliac crests, the great trochanters of the femur, the medial and lateral condyles of the femur, the proximal insertion of the Achilles tendon and insertion of the plantar fascia to the calcaneus. We also assessed how this major entheses index performed in our study group.
Spearman’s correlation was used to determine the relationship between the original MEI and its proposed modifications with disease activity as measured by the BASDAI, the “enthesis question” of the BASDAI, patient and doctor VAS for disease activity, ESR, and CRP. To assess a possible floor effect, the number of patients with a score on the original MEI of >0 was compared with the number of patients with a score on the modified enthesis indexes of >0.
RESULTS
Table 1 presents the patient characteristics and scores on the ASAS core set measures. Of the patients, 112/162 (69%) patients were male, mean duration of complaints was 21.6 years, established disease duration was 11.4 years. Assessment of HLA-B27 was available in 123 patients; of these patients 85% were HLA-B27 positive. Table 2 presents the most frequently scored entheses at baseline, at one year follow up, and at two year follow up. At baseline, the MEI was available for 149 patients, of whom 115 reported a score >0. By pressure on five different entheses, 87 (76%) of these 115 patients were detected. At one year follow up, the MEI was available in 151 patients, of whom 103 reported an MEI >0. By pressure on six different entheses out of 66, 81 (79%) of these 103 patients were identified. At two year follow up, the MEI was available in 129 patients, of whom 86 reported an MEI >0; five entheses identified 71 (83%) of these 86 patients. Of the total of 16 entheses selected at the three measurement periods, five appeared twice, which made a total of 11 selected different entheses. If an enthesis was localised on the left or right side of the body, the enthesis on the contralateral side was also included, which increased the number of entheses to 18. We named this new index the “reduced Mander enthesis index”.
Baseline characteristics and scores on ASAS core set measures of the study patients (mean (SD))
Most frequently scored enthesis points at baseline, at one year follow up, and at two year follow up in order of detection during the data reduction process
To develop an enthesis index which was as concise as possible by further reducing the number of entheses, we excluded the following entheses: L4 because L5 was included; costochondral joint 4, because costochondral joints 1 and 7 were included, and Th1 and Th9 because these are not easy to localise. Moreover L4, Th1, costochondral joint 4, and Th9 were only found in the last steps of the data reduction process and their inclusion increased the percentage of detected patients by only 4% at baseline, 6% at one year follow up, and 8% at two year follow up. The total number of entheses was now reduced to 13. We named this group of 13 entheses the “concise enthesis index”. Table 3 lists the entheses selected in the reduced Mander enthesis index and the concise enthesis index.
Entheses selected in the reduced Mander enthesis index and the concise enthesis index, listed from head to toe
Table 4 presents the number and percentage of patients reporting a score more than 0 on the MEI, the reduced Mander enthesis index, the concise enthesis index, and the major entheses index. At baseline, 24 patients (21%) with a score of >0 on the original MEI were not detected by a score >0 on the concise enthesis index. At one and two years’ follow up these numbers were 20 patients (19%) and nine (10%) patients, respectively. The major entheses index did not detect 32, 42, and 35 patients at baseline, one and two years’ follow up with a score >0 on the MEI (table 5). Table 6 presents the mean, median, 25th–75th centiles, and range of the four different enthesis indices.
Number of patients at baseline, one year follow up, and two year follow up with a score on the Mander enthesis index, the reduced Mander enthesis index, and the concise enthesis index of >0. Results are shown as number (%) of patients
The number of patients missed by the major enthesis index (score = 0), but with a score of >0 on the Mander enthesis index
Values for the Mander enthesis index, reduced Mander enthesis index without gradation, concise enthesis index, and major enthesis index without gradation at baseline, one and two years follow up
To assess a possible floor effect of the MEI, table 7 presents the patients with a score of 0 on the concise enthesis index with a score >0 on the MEI at baseline, one year and two years’ follow up. The total of 53 patients missed with the concise enthesis index predominantly appeared to have low scores on the MEI; 44 of these 53 patients had a score on the MEI of ⩽3 (range 0–90).
The number of patients missed by the concise index (score = 0), but with a score of >0 on the Mander enthesis index
Table 8 presents the correlation between the MEI (with and without gradation), the reduced Mander enthesis index (with and without gradation) the concise enthesis index, and the major entheses index (with and without gradation) and the BASDAI, the enthesis question of the BASDAI, ESR, CRP and the patients and the doctors’ VAS for the degree of disease activity. The enthesis question of the BASDAI did not show a higher correlation coefficient with the MEI (with and without gradation), the reduced Mander enthesis index (with and without gradation), and the concise enthesis index. Furthermore, it can be seen that the correlation with measurements of disease activity does not change significantly when the three different entheses indices are applied. On applying the concise enthesis index without gradation compared with the MEI (which is graded), the correlation was only slightly decreased. We propose to call the concise enthesis index without gradation the “Maastricht Ankylosing Spondylitis Entheses Score” (MASES). Figure 2 shows the entheses of the MASES.
Spearman correlation between Mander enthesis index, reduced Mander index, concise enthesis index with and without gradation versus measures of disease activity
MASES.
DISCUSSION
Some remarks should be made about applying the OMERACT filter to the enthesis index by Mander et al. As far as we know, no reports exist in which the MEI is correlated with histological proof or radiological signs (for example, magnetic resonance imaging (MRI) or ultrasound) of enthesitis. Unfortunately, we were not able to assess this aspect in our study. However, there are studies investigating the correlation between clinical signs of enthesitis (swelling or/and pain) and imaging methods such as MRI or ultrasonography. MRI may show swelling of the enthesis and the peritendinous soft tissue swelling, distension of adjacent bursae by fluid collection, and oedema of the bone near the insertion. Ultrasonography may show the following signs of enthesitis: thickening of the tendon insertion, intratendinous focal changes, calcific deposits in the insertions, and periosteal changes. Recently, Marzo Ortega et al performed a descriptive longitudinal study in which the efficacy of etanercept in the treatment of resistant spondyloarthropathy was assessed in 10 patients.12 In their study clinical enthesitis was investigated by clinical assessment of 78 entheses and by a patient VAS. In the nine patients with clinical enthesitis, clinical enthesitis resolved completely in seven patients and improved in two patients. In these nine patients, there were 44 MRI detectable entheseal lesions, of which 86% resolved completely or improved. The concomitant amelioration of both MRI findings and clinical findings suggests that a clinical measure for enthesitis does indeed depict enthesitis; however, the study was not designed to assess the validity of a clinical measure to assess entheses. Lehtinen et al studied 31 consecutive patients with spondyloarthropathy for the presence of enthesiopathy in the legs both clinically and with ultrasonography.13 Sonography detected inflammatory lesions in 44 entheses of 20 patients, clinical examination detected 56 symptomatic entheses in 20 patients suspected of enthesitis. In 21 entheses, both examinations were positive.
A measure to assess entheses should distinguish enthesitis from other causes of (joint) pain such as arthritis. Mander et al developed their enthesis index in a study in which only patients with AS without peripheral arthritis were included.3 In our patients peripheral arthritis, as measured by the presence of at least one swollen joint, was present in 25% of patients. In the process of developing the MASES, the only enthesis included close to a peripheral joint, was the proximal insertion of the Achilles tendon. Thus less confounding with pain due to peripheral arthritis occurs with the MASES than with the MEI.
An enthesis index is meant to measure the severity of enthesitis. Severity encompasses both the intensity and extent of enthesitis. It is not known whether the patients’ response correlates with the intensity of enthesitis and as stated in the original article by Mander “it is not known whether the severity or simply the presence of tenderness over the entheses is important”.3 Interpretation of the degree of the patients’ response reflected in grades can increase both inter- and intraobserver bias. In joint counts it has been proved that gradation did not improve the performance of the scores and is therefore not recommended.14 Without the gradation, the MEI is easier to perform, and in our study grading did not improve the correlation with measures of disease activity.
The MEI is not very practical to apply clinically because of its extensiveness. This study shows that a reduction of the entheses index to 13 entheses instead of 66 still provides reasonable assessment of the entheses.
On 53 visits, patients who originally had a score on the MEI >0 are missed with the MASES (table 7). Most of these patients had a low score on the MEI. It might be questioned whether a low score on the MEI represents clinically important enthesitis. However, this indicates that there might be some floor effect in the MASES. In our view the non-detection of a few patients is more than balanced by the enormous gain in feasibility. In many trials, including two recently published large trials on non-steroidal anti-inflammatory drugs (NSAIDs),15,16 no measure for entheses is included, illustrating the lack of acceptability of the MEI. This in contrast with the importance placed on entheses by the ASAS group and the opinion that enthesitis can be seen as the cornerstone of the site of inflammation in AS.9,17,18
The fourth question of the BASDAI can be considered to be an enthesiopathy VAS. It might be questioned why this patient VAS is not used to evaluate enthesitis rather than an index used by the doctor. However, it is usual for the doctor to differentiate between entheseal pain and articular, muscular, or other causes of pain; this can be more difficult for patients. Therefore a combination of patient and doctor assessment of enthesitis would be preferable.
The index should discriminate not only between active and inactive disease and between groups of patients but also within individual patients. Only a small study to investigate the discriminative ability of the MEI is reported in the original paper, in which the MEI is compared in patients with AS with and without NSAID treatment. So far we have not tested the discriminative ability of the full MEI, the reduced Mander index, and the MASES. We contacted many authors of published and unpublished trials in AS. None of these had included the MEI in their studies. Therefore we make a plea that the MEI is included in studies so that the discriminative power of the various enthesitis scores can be tested. New trials on tumour necrosis factor blockade treatment could provide this information, especially if MRI of the spine is carried out concomitantly to demonstrate sites of inflammation. While awaiting further validation of the MASES, we recommend its use as it seems to be a good alternative.
Acknowledgments
This study was supported by a grant from the Dutch League Against Rheumatism, grant number NR-99-1-302.