Article Text
Abstract
Objective: To assess the timing of changes in cytokines, cytokine-related markers, autoantibodies and viral antibodies in the pathogenesis of rheumatoid arthritis (RA).
Methods: Case–control study nested in a prospective cohort of 31 330 blood donors in Oslo, Norway. Forty-nine donors developed RA up to 23 years after their most recent blood donation. Stored sera from these donors (case sera) and a sex- and age-matched sample of 245 healthy donors (control sera), and postdiagnostic sera from 33 of the 49 RA cases, were analysed for a panel of cytokines and cytokine-related markers, autoantibodies and antibodies against Epstein–Barr virus and parvovirus B19.
Results: Cytokines and cytokine-related markers were generally negative in case sera from >5 years before the diagnosis of RA. In the 5-year interval immediately before the diagnosis of RA, more case than control sera were positive (odds ratios >2) for interleukin (IL)-1α, IL-1β, IL-1 receptor antagonist, IL-4, IL-10, tumour necrosis factor-α and soluble tumour necrosis factor receptor I. In postdiagnostic sera, however, 11 of 16 examined cytokines and cytokine-related markers were statistically significantly elevated compared with control sera. Seropositivity for IgG antibodies against cyclic citrullinated peptides and for IgM and IgA rheumatoid factors were seen in case sera from up to 18 years before the diagnosis of RA. IgG antibodies against Epstein–Barr virus and parvovirus B19 did not differ significantly between case and control sera.
Conclusions: Cytokines and cytokine-related markers appear to be upregulated rather late in RA pathogenesis. In contrast, IgM rheumatoid factor and IgG anti-cyclic citrullinated peptide autoantibodies may precede the diagnosis of RA by up to two decades.
Statistics from Altmetric.com
Rheumatoid arthritis (RA), the most common of the autoimmune rheumatic diseases affecting approximately 0.5–1% of Western populations, is a systemic disorder characterised by the persistence of synovial inflammation. Without treatment, RA often progresses with destructive erosion of cartilage and bone brought about by locally expressed cytokines and degradative matrix metalloproteinase enzymes.1–6
Early treatment with disease-modifying antirheumatic drugs (DMARDs) within 3 months after disease onset has been shown to counteract disability and retard radiographic progression. Also, initiation of preventive measures or DMARD treatment even before the onset of arthritis may minimise irreversible joint damage.7 8 Thus, efforts to identify early biomarkers associated with RA risk are clearly warranted.9–12
Autoantibodies, immune complexes and an imbalance in the cytokine network play central roles in the initiation of RA.9 Cytokines are signal peptides that also play a key role in RA pathology. Upregulated cytokine profiles in patients with RA are distinct from those of healthy individuals, and cytokine profiles may differ between patients with RA with recent disease onset and those with established disease.13 However, little is known about the timing of premorbid changes in cytokine profiles in people destined to develop RA. Insight into such premorbid changes might be useful in future attempts to target at-risk individuals and provide DMARD treatment or prediagnostic advice as to avoid risk factors and behaviour that may precipitate RA.14
Two major cytokines, tumour necrosis factor (TNF)-α and interleukin (IL)-1 have received particular attention as central actors in RA-associated chronic inflammation. However, the list of cytokines, including chemokines, and other mediators putatively involved in RA has expanded considerably in recent years.5
Production of autoantibodies such as rheumatoid factors and antibodies against citrullinated peptides and proteins precedes the development of clinical manifestations in RA by several years.15–23 Rheumatoid factors are autoantibodies directed against the Fc component of normal immunoglobulin G while anti-citrulline peptide antibodies target antigens containing the unusual amino acid citrulline. Anti-citrulline peptide antibodies have received particular attention in recent years because they are highly specific and sensitive markers of RA.19 20 24 25 The use of a cyclic citrullinated peptide (CCP) as a surrogate autoantigen for demonstrating anti-citrulline peptide antibodies has been proven to cover the majority of anti-citrulline antibodies.26 Recent studies strongly suggest that the presence or absence of anti-CCP antibodies in serum may divide RA into two distinct categories with separate aetiologies and clinical courses.14 However, so far only few studies have examined anti-CCP antibody levels in premorbid sera from patients with RA.19 20 25
Furthermore, patients with RA have been shown to have higher systemic loads of Epstein–Barr virus (EBV) than healthy controls and infectious human parvovirus B19 has been located in synovial tissue of patients with RA. Thus, infectious agents could also contribute to RA pathogenesis.27–32
MATERIALS AND METHODS
Study cohort
We conducted a case–control study nested in a prospective cohort of 31 330 blood donors in Oslo, Norway, who had blood samples collected between 1973 and 2000 and stored at –25°C in the JANUS Serum Bank.33 Our study comprised a total of 49 premorbid sera from donors (cases) who later developed RA (31 women, 18 men) according to the American College of Rheumatology 1987 classification criteria.34 These donors were identified as RA cases through data linkage with the Oslo Rheumatoid Arthritis Registry35 by the unique 11-digit personal identifier assigned to every Norwegian citizen. Serum samples collected after disease onset were obtained from the Oslo Rheumatoid Arthritis Registry for 33 of the 49 cases (20 women, 13 men). Information about the time of the diagnosis of RA was obtained from the Oslo Rheumatoid Arthritis Registry.
Each case was individually matched on sex, birth date, number of blood donations before the year of RA diagnosis, date for earliest blood donation and, when relevant, date for most recent blood donation with five other blood donors in the cohort who were disease-free (ie, without RA or cancer) at the time of the diagnosis of RA in the corresponding case. A total of 245 sera from control subjects (155 women, 90 men) were analysed. We first chose eligible controls who were close (<0.5 year) in birth date, date of first and date of most recent blood donation. However, in order to obtain five controls per RA case, we gradually broadened these criteria. Thus, for 242 (99%) of the 245 finally included controls the birth date, date of first blood donation, and date of most recent blood donation differed by up to 3 years from the corresponding dates in the matched case. The final three controls were up to 5, 6½ and 8 years younger or older than their corresponding case but equally closely matched on dates of first and most recent blood donations (±3 years). Additional premorbid serum samples were available for analysis from 33 of the 49 RA cases. Because all patients with a first sample testing positive for IgM rheumatoid factor or IgG anti-CCP antibodies also had a subsequent positive sample we restricted all analyses to the most recent serum sample available.
Serological testing
Serological testing took place in laboratories at Statens Serum Institut, Copenhagen, Denmark, and at the Institute for Inflammation Research, Rigshospitalet, Copenhagen, Denmark. Each serum sample was tested for a range of: proinflammatory cytokines (IL-1α, IL-1β, IL-2, IL-5, IL-6, IL-8, IL-12p70, TNF-α, interferon (IFN)-γ and granulocyte-macrophage colony-stimulating factor); anti-inflammatory cytokines (IL-4 and IL-10); a cytokine receptor antagonist (IL-1RA); an anti-cytokine autoantibody (anti-IL1α-IgG); soluble TNF receptors (sTNF-RI, sTNF-RII); autoantibodies (IgM and IgA rheumatoid factors, IgG anti-CCP); antibodies against Epstein–Barr virus (anti-EBNA IgG and anti-EBV-VCA IgG) and anti-parvovirus B19 IgG. Samples were defined in this study as cytokine positive if titres were above the 95th percentile of titres among control subjects. Owing to unforeseen conjugate shortage, IgA rheumatoid factor was only analysed for case sera.
Serum analyses
Quantification of cytokines and cytokine receptors
Plasma levels of the cytokines and soluble cytokine receptors were quantified with a Luminex 100 IS (Luminex Corp., Austin, TX, USA), using StarStation v. 2.0 software (Applied Cytometry Systems, Sheffield, UK) for acquisition and analysis. The reagents were purchased from Biosource, Neuville, Belgium (Human Cytokine Panel I multiplex kit, measuring IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, TNF-α, IFN-γ and granulocyte-macrophage colony-stimulating factor, and singleplex kits for IL-1RA, IL-12p70, sTNF-RI and sTNF-RII) and from Upstate, Trichem, Frederiksund, Denmark (singleplex kit for human IL-1α). As the detection antibodies to sTNF-RI and IL-1RA interfered with other analytes, resulting in unacceptable background levels of certain cytokines, each sample was tested using a two-plex assay (sTNF-RI and IL-1RA) and an additional 13-plex assay.
To ensure that the standard used for quantification of cytokine levels resembled the project samples as much as possible, we prepared an in-house standard by collecting 20 ml blood from two healthy female and male blood donors in Li-Heparin tubes (BD Vacutainer, BD, Brondby, Denmark). One part from each was diluted five times in 1640 RPMI (Biochemical Industries, Kibbutz Beit Haemek, Israel), and the supernatant was frozen until use (control plasma). The other parts were diluted in 1640 RPMI as above and stimulated with 0.1 μg/ml lipopolysaccharide (Bacto LPS, BD) plus 20 μg/ml phytohaemagglutinin (BD Difco, BD, Brondby, Denmark) for 24 h at 37°C. The cell-free supernatants were collected, split into aliquots, and stored at −20°C until further use (cytokine-rich plasma). The levels of cytokines and soluble cytokine receptors in these in-house standards were calibrated using WHO International Standards (National Institute for Biological Standards and Control, Hertfordshire, UK).
Cytokines were quantified using the Biosource protocol for plasma samples in 96-well filter plates (Millipore; MSBVN1210, Glostrup, Denmark). The calibrated cytokine-rich plasma was used as standard, and control plasma and a cytokine-rich plasma sample were analysed on each plate as inter-plate controls.
Quantification of autoantibodies to interleukin-1α
Autoantibodies to IL-1α were quantified by radioimmunoassay as previously described.36 The results are given as maximum displaceable binding in per centage of added rIL-1α.
Quantification of rheumatoid factors and anti-cyclic citrullinated peptide antibodies
IgM rheumatoid factor was quantified by an ELISA method using purified human IgG as antigen37 with a positive cut-off value of 17 IU/ml. IgA rheumatoid factor was determined by an ELISA using highly purified human IgG to avoid IgA contamination, but otherwise used the same methodology, with a positive cut-off value of 25 AU/ml. IgG anti-CCP antibodies were quantified using a commercial ELISA (Immunoscan RA, from Eurodiagnostica, Malmö, Sweden) with a positive cut-off of 25 AU/ml.
Quantification of antibodies against Epstein–Barr virus and parvovirus B19
Epstein–Barr virus EBNA and VCA IgG antibodies were measured using two kits, Anti-EBV EBNA IgG ELISA and Anti-EBV VCA IgG ELISA, from Biotest AG (Dreieich, Germany). Parvovirus B19 IgG antibodies were measured using the Parvovirus B19 IgG Enzyme Immunoassay kit from Biotrin International Ltd (Dublin, Ireland). All tests were performed in accordance with the manufacturers’ instructions.
Statistical analyses
Associations between the biomarkers under study and RA risk were measured by odds ratios (OR) estimated in exact conditional logistic regression analyses taking the individual case–control matching procedure into account, and 95% confidence intervals (CIs) were estimated by mid-p CIs.38 ORs were estimated by a median unbiased estimator when no samples tested positive for a given marker,39 and ORs were presented as zero when no case samples tested positive for a given biomarker.
No difference in sex distribution was found with respect to the prevalence of positivity for any of the measured cytokines, cytokine-related markers or autoantibodies in either premorbid or postdiagnostic patient samples, so all analyses were carried out on the combined material of male and female participants. All p values are two-sided. p<0.05 and ORs with 95% CIs, excluding unity, were considered statistically significant. All calculations were performed using SAS software, V.9.1.
Ethics
The study was approved by the regional ethical committee, the Norwegian Data Inspectorate, the Steering Committee of the JANUS Serum Bank and the scientific board of the Norwegian Cancer Society, who was the formal owner of the JANUS Serum Bank until 2004 (today the JANUS Serum Bank is owned by the Norwegian Cancer Registry). No patient contact took place in this study.
RESULTS
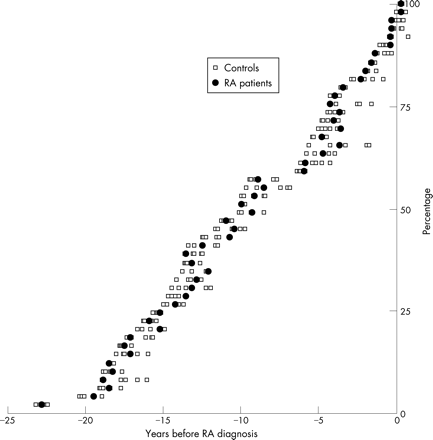
The median age for patients with RA (cases) at the time of premorbid blood sampling was 41 (range 21–64) years, and their median age when the RA was diagnosed was 50 (range 24–83) years. The median time between blood sampling and fulfilment of American College of Rheumatology 1987 classification criteria for RA was 9.3 (range 0.3–23) years (fig 1).
Cytokines and cytokine-related markers
Overall, cytokine levels in premorbid case sera were not systematically different from those of control sera (table 1). No cytokine or cytokine-related marker was found to be positive in more than 7% of case sera from the period >5 years before the diagnosis of RA. Seven cytokines or cytokine-related markers (IL-1α, IL-1β, IL-1RA, IL-4, IL-10, TNF-α and sTNF-RI) had ORs >2 in sera from ⩽5 years before the diagnosis of RA (table 2), although this association was only statistically significant for TNF-α (OR 12.4; 95% CI 1.3 to 335). Eleven of 16 (69%) of the cytokines or cytokine-related markers that were examined were statistically significantly elevated in sera collected after the diagnosis of RA (table 2).
Autoantibodies
Premorbid case sera were significantly more likely to be IgG anti-CCP positive (31%), IgM rheumatoid factor positive (20%) or both (14%), compared with sera from control subjects, 1%, 3% and 0%, respectively (table 3). Corresponding ORs were 37.5 (95% CI 9.8 to 242) for IgG anti-CCP, 7.5 (95% CI 2.7 to 22.6) for IgM rheumatoid factor and 48.0 (95% CI 9.4 to ∞) for positivity for both autoantibodies. The proportion of IgG anti-CCP seropositive case sera increased as the time of RA diagnosis approached, rising from 17% in samples from >5 years before RA diagnosis to 53% in samples from ⩽5 years before RA diagnosis, remaining high in postdiagnostic samples (55%). For IgM rheumatoid factor there was no apparent change over time in the proportion of seropositive cases before RA diagnosis, being 20% and 21% in sera from >5 years and ⩽5 years before RA diagnosis, respectively; however, the proportion of postdiagnostic case sera that were seropositive increased to 64%. For IgA rheumatoid factor the proportion of seropositive cases increased from 14% in sera from >5 years before to 32% ⩽5 years before RA diagnosis, and remained high (34%) in postdiagnostic sera (table 3). The longest interval before RA diagnosis that we observed seropositivity in case sera for IgG anti-CCP, IgM rheumatoid factor and IgA rheumatoid factor was 18, 17 and 17 years, respectively.
Epstein–Barr virus and parvovirus B19 antibodies
Seropositivity for IgG antibodies against Epstein–Barr virus was somewhat less common in case sera than control sera, though not significantly so (table 3). This applied to both EBNA and VCA antibodies and to both premorbid and postdiagnostic serum samples. Likewise, there was no indication that the rate at which parvovirus B19 IgG antibodies occurred was unusual in individuals destined to develop RA (table 3).
DISCUSSION
In accordance with prevailing knowledge our study shows that patients with established RA are characterised immunologically by having an upregulated cytokine profile and significantly elevated levels of RA-associated autoantibodies.
Additionally, seven of the 16 cytokines or cytokine-related markers that were examined, IL-1α, IL-1β, IL-1RA, IL-4, IL-10, TNF-α and sTNF-RI, were significantly upregulated postdiagnostically and had ORs >2 during the ⩽5 year period before the diagnosis of RA. However, of these only IL-1α, IL-1β, IL-1RA, IL-4 and TNF-α were positive in at least two of the 19 premorbid case samples collected during that interval. ORs were <2 for all cytokines and cytokine-related markers in samples from >5 years before the diagnosis of RA.
The proinflammatory cytokine TNF-α is particularly important in initiating and maintaining the inflammatory process in RA, and TNF-α blocking therapy has gradually become an integrated and important element in RA treatment, helping a large proportion of patients through a less aggressive disease course and, in some cases, improving on previously induced structural joint damage.3 40 In this study three patients with RA, all women, who were TNF-α positive before RA diagnosis had their analysed samples drawn 0.3, 3.5 and 4.0 years before RA diagnosis, whereas TNF-α levels were undetectable in all samples from >5 years before RA diagnosis. Data from the Oslo Rheumatoid Arthritis Registry showed that the three cases with premorbid serum samples testing positive for TNF-α were all rheumatoid factor positive and had a progressive and erosive disease course. Despite normal or only marginally increased levels of acute phase reactants, all three had persistent active polyarthritis and have been treated with methotrexate, and one with anti-TNF drugs. The proinflammatory cytokine IL-1 has also been associated with RA.40–42 Both of its molecular forms, IL-1α and IL-1β, and the anti-inflammatory IL-1 antagonist IL-1RA seemed to undergo upregulation in the 5 years immediately before RA diagnosis. Thus, both pro- and anti-inflammatory responses appear to be upregulated before RA diagnosis, but this appears to be a rather late occurring event in the pathogenesis of the disease.
As expected, IgG anti-CCP antibodies were strongly associated with RA risk and may antedate RA by many years. Approximately one in six patients with RA had detectable anti-CCP antibodies >5 years prior to RA diagnosis. This proportion increased to 53% in the interval ⩽5 years before diagnosis while, among controls, the prevalence remained low at 0% and 2%, respectively, in the same time intervals. Our results are in agreement with other recent findings of premorbid serological changes in patients with RA.19 20 25 In 33 sera from patients with RA taken within 1.5 years before RA onset, Rantapää-Dahlqvist et al found 52% to be anti-CCP antibody positive, 30% IgM rheumatoid factor positive and 39% IgA rheumatoid factor positive.20 When testing multiple premorbid blood samples from 79 patients with RA Nielen et al19 found comparable prevalence estimates for anti-CCP antibodies and IgM rheumatoid factor (40.5% and 27.8%, respectively). Combined with our seropositivity rates of 53% for IgG anti-CCP, 21% for IgM rheumatoid factor and 32% for IgA rheumatoid factor up to 5 years before RA diagnosis, we suggest that IgM and IgA rheumatoid factors and, most notably, IgG anti-CCP antibodies may be useful biomarkers to identify individuals at high risk of developing RA, thus making early prevention or therapy possible and, hopefully, more effective.12 The combined presence of anti-CCP and IgM rheumatoid factor antibodies (21% of case sera from ⩽5 years before RA diagnosis versus 0% of control sera overall in this study) appears to be a particularly specific marker for the development of RA.
It has been speculated that infectious agents may be involved as possible triggers of RA. In the present study, premorbid serum samples from donors destined to develop RA were slightly less likely to be positive for IgG antibodies against two Epstein–Barr virus antigens than sera from healthy controls, and this non-significant deficit of EBV antibodies was seen in both premorbid and postdiagnostic sera from the patients with RA. This finding was not anticipated because patients with RA in other studies have been found to have elevated EBV-DNA titres.27 28 Also, our data do not support a previously reported positive association between RA and infection with parvovirus B19.30–32 Taken together, our findings fail to support the view that EBV or parvovirus B19 are important viral triggers of RA development.
Two premorbid serum samples were available for analysis in 33 of the 49 RA cases. All four patients that had a first sample positive for IgM rheumatoid factor or IgG anti-CCP antibodies also had a subsequent positive sample. Seven patients with a first negative premorbid serum sample for IgG anti-CCP antibodies had a second premorbid serum sample testing positive. Thus, IgG anti-CCP antibody testing status may be repeated in previously anti-CCP negative individuals at risk of RA (eg, individuals with a family history of RA). Cytokine levels, however, were somewhat less stable over time. Overall, for patients with RA who had two premorbid samples, only 26% of those who had a first premorbid sample that tested positive for a cytokine or cytokine-related marker also had a more recent premorbid sample testing positive for the same marker. We arbitrarily defined cytokine positivity as levels above the 95th percentile of control levels. Possibly, the observed seroconversions from first to second premorbid serum sample might reflect the lack of well-established cut-off values to define positivity for cytokines and cytokine-related markers. Alternatively, we cannot exclude that some of the observed elevated cytokines and cytokine-related markers may be the result of chance, considering the limited number of sera available in our study and the large number of tests performed.
Our study had limited statistical power due to the low number of blood donors in the study cohort who subsequently developed RA. Thus, our findings need to be interpreted cautiously and larger and more powerful studies with more participants are clearly needed to confirm and expand our findings on cytokines and cytokine-related markers. Nevertheless, our novel results are in accord with those of Nielen et al who found a rise in the inflammatory markers, C-reactive protein and soluble phospholipase A2, in the preclinical phase of RA with levels most notably increased within 2 years before the onset of RA symptoms.25 43
In summary, our study suggests that a number of cytokines and cytokine-related markers undergo upregulation in the 5 years before RA diagnosis. TNF-α appears to be a proinflammatory cytokine of particular importance. Indeed, our study provides the first prospective evidence to justify using TNF-α-blocking medications in the management of patients with early RA. Also, our study provides prospective epidemiological evidence that particularly IgG anti-CCP antibodies, but also IgA and IgM rheumatoid factors are strongly associated with a risk of developing RA and may antedate RA diagnosis by almost two decades. Early diagnosis and treatment with DMARDs are already essential parts of the treatment strategy for patients with recent-onset RA. The current evidence of anti-CCP antibodies as a predictor of subsequent development of persistent arthritic disease suggests that DMARD treatment of anti-CCP-positive individuals without arthritis should be considered as a next step on the research agenda to retard or even prevent the development of clinically manifest RA.
REFERENCES
Footnotes
Funding: The study has been supported by unrestricted research grants from The Danish Rheumatism Association, The Danish Medical Research Council, Health Insurance Denmark Science Foundation, Aase and Ejnar Danielsen’s Foundation, The Gangsted Foundation, Dagmar Marshall’s Foundation, Krista and Viggo Petersen’s Foundation, Frederik Leth Christiansen’s Foundation, Lily Benthine Lund’s Foundation, Kurt Bønnelycke and Mrs Grethe Bønnelycke’s Foundation, Hede Nielsen’s Foundation, Poul Martin Christiansen’s Foundation, The Linex Foundation, Apotekerfonden and the Frimodt-Heineke Foundation, Director Ib Henriksen’s Foundation, Henny and Helge Holgersens Memorial Fund, Torben and Alice Frimodt’s Foundation, Max Fodgaard’s Foundation, Niels Hansen and Wife’s Foundation and Frode V. Nyegaard and Wife’s Foundation.
Competing interests: None.