Article Text
Abstract
Objective: To assess mortality in patients with rheumatoid arthritis (RA) treated with tumour necrosis factor (TNF) inhibitors, compared with a standard RA population.
Methods: Patients were recruited from a regional register, which includes over 90% of patients with RA treated with TNF blockers in the area in 1999 or later, and a local community-based cohort of patients with RA, established in 1997. Of a total of 1430 patients in the combined cohort <80 years old, 921 received treatment with TNF inhibitors during the study period. The total cohort was linked with the national register for cause of death. Overall mortality in those treated versus those not treated with TNF blockers was estimated using standardised mortality ratios and time-dependent Cox proportional hazards.
Results: There were 188 deaths per 7077 person-years at risk in the total cohort. Controlling for age, sex, disability and baseline comorbidity, the adjusted HR for death was 0.65 (95% CI 0.46 to 0.93) in those treated with anti-TNF versus those not treated. The effect was significant in women (HR = 0.52, 95% CI 0.33 to 0.82) but not in men (HR = 0.95, 95% CI 0.52 to 1.71).
Conclusion: After adjusting for disease severity, treatment with TNF inhibitors was found to be associated with a reduced mortality in women but not men with RA. These findings are compatible with a critical role for inflammation in RA-associated premature mortality.
- COPD, chronic obstructive pulmonary disease
- CVD, cardiovascular disease
- DMARD, disease-modifying antirheumatic drug
- HAQ, Health Assessment Questionnaire
- ICD, International Classification of Diseases
- PYRS, person-years
- RA, rheumatoid arthritis
- SMR, standardised mortality ratio
- SSATG, South Swedish Arthritis Treatment Group
- TNF, tumour necrosis factor
- VAS, visual analogue scale
Statistics from Altmetric.com
- COPD, chronic obstructive pulmonary disease
- CVD, cardiovascular disease
- DMARD, disease-modifying antirheumatic drug
- HAQ, Health Assessment Questionnaire
- ICD, International Classification of Diseases
- PYRS, person-years
- RA, rheumatoid arthritis
- SMR, standardised mortality ratio
- SSATG, South Swedish Arthritis Treatment Group
- TNF, tumour necrosis factor
- VAS, visual analogue scale
Rheumatoid arthritis (RA) is a chronic inflammatory disease, which, in many patients, leads to a substantial disability and has a major effect on the quality of life. Patients with RA also have an increased mortality compared with the general population,1–,3 mainly due to increases in mortality from cardiovascular disease (CVD)1,4 and infections.5 Established risk factors for premature mortality include major inflammation,2 disability6 and severe extra-articular disease manifestations.7 It would seem reasonable that effective treatment with disease-modifying antirheumatic drugs (DMARDs) might decrease the risk of comorbidity and premature mortality, and this concept has been supported by observational studies on patients with RA treated with methotrexate.8,9
Tumour necrosis factor alpha (TNFα) is an important proinflammatory cytokine, abundantly expressed in synovitis in RA.10 It is also of importance for immune surveillance of infections11 and malignancies,12 and is of demonstrated importance in unstable arteriosclerotic plaques.13 In recent years, several randomised controlled trials with TNFα blockers14–,16 have shown efficacy in reducing inflammation and joint destruction in RA. On the other hand, there have been concerns about potential side effects, including comorbidities. Theoretically, immune suppression could increase the risk of severe infections and malignancies,11,12 but effective DMARD treatment may also decrease the risk by reversing some features of immune dysregulation associated with active RA.17,18 The net effect of this on RA-associated comorbidities is unknown. We have recently demonstrated that the rate of new-onset CVD is lower in patients treated with TNF inhibitors compared with other patients with RA,19 suggesting that blocking TNF may have a beneficial effect on arteriosclerosis. The impact of TNF inhibition on the overall mortality in patients with RA, and to what extent this depends on age, sex and disease characteristics, has not been studied extensively.
The aim of this study was to estimate the relative risk (RR) for overall mortality in patients with RA treated versus those not treated with anti-TNF.
PATIENTS AND METHODS
Study design
This study is based on an estimation of the total mortality risk in a community-based register of patients with RA treated with TNF blockers and in a community-based comparison cohort of patients with RA within the same geographical area. In the present analyses, the two cohorts were treated as one, and the effects of TNF blockers and other risk factors for mortality—that is, markers of disease severity—were evaluated in a time-dependent fashion. Information on events was obtained from national registers for this combined cohort.
The TNF inhibitor exposed group
The South Swedish Arthritis Treatment Group (SSATG) register has been described previously.20 The catchment area for the register is approximately 1 300 000 inhabitants. The SSATG register includes patients with RA treated with leflunomide, anti-TNF drugs, anti-interleukin 1 and other new DMARDs at 10 rheumatology units. The register has been compared with pharmaceutical sales data and found to cover over 90% of patients treated with anti-TNF in the area.20 Patients with RA according to a rheumatologist treated with TNF inhibitors and included in the SSATG register between 1 February 1999 and 31 December 2002 (n = 949) were studied. Patients treated with interleukin 1 inhibitor were excluded from the analyses.
Patient and disease characteristics including age, sex, disease duration, Health Assessment Questionnaire (HAQ),21 visual analogue scale (VAS) for patient global assessment of disease severity (VAS global assessment) and pain (VAS pain), respectively, and data on previous DMARD medication, registered at inclusion, were retrieved from the register for the purpose of this analysis. Follow-up of these patients began when anti-TNF treatment was first initiated (after 1 February 1999), with the exception of the subgroup that was already a part of the comparison group, which was analysed in a time-dependent fashion. Only subjects <80 years old at baseline (n = 921) were included in the study.
The comparison group
In 1997, a register of all known patients with RA in Malmö (the major city of Region Skåne), Sweden, was established. Inclusion was based on a clinical diagnosis of RA by a rheumatologist and fulfilment of the 1987 American College of Rheumatology criteria for RA.22,23 Subsequent surveys, using the diagnostic index of primary care centres and questionnaires sent to other physicians in the area, indicate that >90% of all patients with diagnosed RA in the city have been seen by a rheumatologist and thus are included in the register.23 In the 1997 survey, a total of 1016 patients with RA were identified, corresponding to a prevalence of patients with RA currently under active care of 0.49% in the adult population—close to a recent prevalence estimate from Oslo, Norway.24 In July 1997, these patients were sent a questionnaire, which was answered by 734 (72%) patients. The questionnaire was used to obtain data on the time of onset of RA, present and previous treatment with DMARD and prednisolone, HAQ, VAS global and VAS pain. Follow-up of this subset began on 1 July 1997. In the subgroup that later received anti-TNF treatment, information regarding these factors at the start of such treatment was also used in the analyses in a time-dependent fashion. Only subjects <80 years old at baseline (n = 652) were included in the study.
Event definition and statistical analysis
Data on vital status were retrieved from Statistics Sweden (Swedish Cause of Death Register) up to 31 December 2004. Standardised mortality ratios (SMRs) and SMR ratios were computed using these national Swedish mortality and census data for the same time period, adjusting for calendar year, age and sex.
Baseline comorbidities were assessed using the Swedish national hospital discharge register,25 which has a nationwide coverage from 1987. It includes information on age, sex and place of residence for each individual, as well as the time of hospitalisation and discharge, and discharge diagnoses, classified according to the International Classification of Diseases (ICD-9 and ICD-10)26,27 for each inpatient episode. The proportion of hospital discharges not reported to the register has been estimated to be 1–2%,28 and the validity of discharge diagnoses is almost 90%.29 To be able to adjust for prevalent comorbidities, any record of hospitalisation between 1987 and the date when an individual entered this study was retrieved. If a person had been hospitalised and given a diagnosis of CVD (including coronary artery disease (ICD-9: 410; ICD-10: I200–I219), congestive heart failure (ICD-9: 434 or 436; ICD-10: I500–I509) and cerebrovascular disease (ICD-9: 413; ICD-10 I630–I649)), diabetes (ICD-9: 250; ICD-10: E100–E140) or chronic obstructive pulmonary disease (COPD) (ICD-9: 491C or 492X; ICD-10: J440–J449) before inclusion, he or she was categorised as having these comorbidities throughout the study period.
For those in the comparison group the period of risk began at the time of response to the questionnaire (1 July 1997) and for those in the TNF inhibitor-exposed group when they started receiving TNF blockers for the first time (after 1 February 1999 when these drugs became available in Sweden) and were included in the SSATG register. Subjects were censored at death or at the close of the study, whichever occurred first. For assessment of overall death risk, the data on death dates were available up to 31 December 2004, which was set as the closing date for these analyses. Data on cause-specific deaths were available only up to 31 December 2002 and were therefore not included in the analyses.
To assess mortality ratios, proportional hazards regression models were used. Age at inclusion, sex and presence of comorbidity (CVD, diabetes or COPD) were used as non-time-dependent covariates. Anti-TNF treatment (yes or no) and markers of disease severity such as disease duration, HAQ, VAS pain, VAS global assessment, number of previous DMARDs and present prednisolone treatment (yes or no) were used as covariates in a time-dependent manner. That is, in subjects who were not exposed to anti-TNF (ie, belonging to the comparison group) at the start of the study, and who received this treatment during the course of the study, new values for these risk factors were incorporated into the model as they started such treatment. Apart from this exception, only information from when the patients were first included in the registers was used. Individuals were considered as exposed to anti-TNF from the start of such treatment to the end of the study. Age and sex/HAQ-adjusted survival curves were used to illustrate the survival rates by anti-TNF exposure, sex and HAQ.
Of the total 1430 patients (table 1⇓) in the combined cohort, 921 were part of the SSATG cohort and 652 were part of the comparison cohort. Thus, 143 patients from Malmö were started on anti-TNF treatment and included in the SSATG. These patients were included in both groups and contributed with person-years (pyrs) at risk to both those exposed and those not exposed to anti-TNF treatment.
Patient characteristics at the start of the study for all subjects and when first entering as anti-TNF exposed and not exposed respectively
RESULTS
Table 1⇑ provides the median age, sex distribution and disease characteristics for the whole unified cohort. In addition, similar information is given for those exposed to and those not exposed to anti-TNF treatment by sex, as they first started their follow-up in the respective groups (table 1⇑). Patients treated with anti-TNF drugs had higher markers of disease severity and a history of more extensive treatment with DMARDs and prednisolone. Baseline comorbidities, defined as previous hospitalisation for COPD, diabetes or CVD, were less frequent in the anti-TNF-treated group, which however was also younger and had a larger proportion of females (table 1⇑). These factors were adjusted for in subsequent analyses.
Total mortality (up to 31 December 2004)
There were 188 deaths per 7077 pyrs at risk in the total cohort. Of these deaths, 63 occurred in men (per 1817 pyrs at risk) and 125 (per 5260 pyrs at risk) in women (table 2⇓).
Number of deaths, mortality with standardised mortality ratios (SMRs) and SMR ratios using Swedish national data as reference population
Mortality was compared with Swedish national mortality rates calculating the SMRs by anti-TNF exposure and gender. After 2, 3 and 4 years of follow-up, the SMRs were close to 1 or higher for both men and women, irrespective of anti-TNF exposure. The SMR ratios, comparing those who were exposed to anti-TNF with those who were not, were found to be consistently below 1 in women. The SMR ratio estimates for men were consistently related after 2, 3 and 4 years of follow–up (table 2⇑).
Using time-dependent proportional hazards models in the combined cohort, disability, reflected by a high HAQ score, was found to be a strong predictor of mortality, adjusted for age and sex (adjusted hazard ratio (HR) 1.58/1.66; 95% CI 1.37 to 1.82). Higher VAS scores for pain, patient global disease assessment and the presence of comorbidity (COPD, diabetes or CVD) were also significant predictors of mortality. Similar point estimates were found for men and women (table 3⇓).
HRs (95% CIs) for total mortality for various possible predictors for all subjects and by gender
There were 51 deaths per 3177 pyrs among those treated with TNF inhibitors compared with 137 deaths per 3900 pyrs among those not treated. Anti-TNF treatment was associated with a lower mortality in models adjusted for age, sex and disability (table 3⇑). Adjustment for other disease severity measures in addition to HAQ had no major effect on this association (data not shown). The lower mortality also remained significant when controlling for age, sex, disability and comorbidity (adjusted HR 0.65; 95% CI 0.46 to 0.93). The effect of TNF blockers on mortality interacted with gender (fig 1⇓). When controlling for age, disability and comorbidity, mortality was significantly reduced in women (adjusted HR 0.52; 95% CI 0.33 to 0.82) but not in men (adjusted HR 0.95; 95% CI 0.53 to 1.71) (table 3⇑).
Age–Health Assessment Questionnaire score-adjusted survival curves for patients treated versus those not treated with tumour necrosis factor (TNF) inhibitors, by sex.
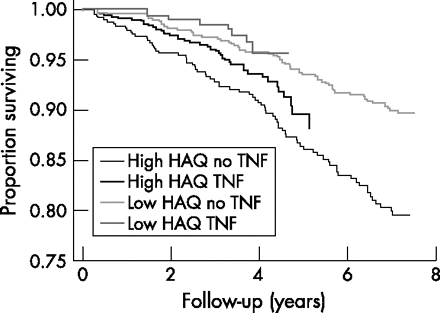
Anti-TNF treatment and the level of HAQ did not interact with regard to their effect on mortality. When stratifying by the median of the HAQ score and adjusting for age and sex, TNF blockers were found to have a major effect on mortality in those with higher as well as in those with lower disability scores (fig 2⇓). The lowest mortality risk was found in those who had a low HAQ score at baseline and were treated with TNF inhibitors (fig 2⇓). When controlling for age, sex and comorbidity, mortality was significantly reduced in both those with an HAQ value below the median (adjusted HR 0.64; 95% CI 0.28 to 1.46) and those with an HAQ value above the median (adjusted HR 0.61; 95% CI 0.40 to 0.92).
Age- and sex-adjusted survival curves for patients treated versus those not treated with tumour necrosis factor (TNF) inhibitors, and stratified by the median Health Assessment Questionnaire (HAQ) score for each sex separately (median HAQ for men 1.13 and for women 1.50), in the total cohort at inclusion.
To assess whether the first period of follow-up introduced any channelling bias, RRs for mortality adjusted for age, sex, disability and comorbidity were assessed omitting the first 6 and 12 months of follow-up. When doing this, HR point estimates were 0.67 (95% CI 0.46 to 0.96) and 0.70 (95% CI 0.61 to 1.03), respectively.
To address the issue of differences in any channelling bias due to the alert given by the Food and Drug Administration (October 2001), regarding the possible danger of using TNF inhibitors in patients with severe congestive heart failure, HRs were computed separately for patients receiving such treatment before and after this date. Similar point estimates were found (before: adjusted HR 0.70, 95% CI 0.48 to 1.01; after: adjusted HR 0.67, 95% CI 0.45 to 0.95).
DISCUSSION
In this study, we found a lower risk for mortality in patients with RA treated with TNF blockers compared with those not treated when controlling for markers of disease severity. The reduced mortality was mainly seen in women. To our knowledge, this is the first study to evaluate the effect of TNF blockers on mortality.
Premature mortality in RA is predicted by disease severity,2,6,7,30 CVD morbidity and mortality, specifically by severe extra-articular RA30 and persistently high disease activity.31 In accordance with this, we found increased disability and disease severity (VAS scores and extent of previous DMARD intake) to predict overall mortality as well as mortality from CVD (data not shown). Furthermore, we demonstrated that patients starting anti-TNF treatment had a higher disability and more severe disease compared with a community-derived RA population and thus are likely to have a higher baseline risk for death, especially from CVD. We suggest that excess mortality in patients with RA, independent of initial disability, is reduced by aggressive antirheumatic treatment. This is also supported by the findings of Choi et al,9 who demonstrated that treatment with methotrexate for RA was associated with a reduced risk of cardiovascular mortality in a large observational study, and our own data, which demonstrated a decreased risk for first-time CVD events after start of anti-TNF treatment.19 As expected, the majority of deaths analysed through 2002 in this study (data not shown) were caused by cardiovascular disease.
The protective effect of TNF inhibitors on mortality was significant in women, but not in men. Previous studies have suggested that excess mortality compared with the background population in well-defined patient samples is greater in women with RA than in men,3 and that women may have more pronounced disease-associated abnormalities, such as increased arterial stiffness.32 Our data suggest that the potential gain in reducing comorbidity and mortality by TNF inhibition is greater in female patients. The lack of protective effect of TNF blockers in men may also to some extent be influenced by a higher mortality in the male comparator group, resulting in a tendency for the HR estimate to be closer to 1. Due to the lower number of men with RA, the point estimates are also less precise in men compared with women.
Immunological abnormalities associated with RA are likely to be pathophysiologically involved in the development of comorbidities, and these could be modified by treatment. For example, the presence of rheumatoid factor predicts premature mortality in the general population,33,34 and clonal expansion of CD4+ CD28null T cells with natural killer capabilities has been demonstrated in both patients with severe RA35,36 and patients with coronary artery disease.37 Recent studies suggest that such abnormalities are improved in responders to TNF inhibitors.18,37,38 Furthermore, in the ApoE−/− knockout arteriosclerosis mouse model, further genetic modification with a TNF gene knockout, or continuous treatment with a soluble TNF receptor, led to reduced arteriosclerosis.39 These findings suggest that several immunomodulatory and anti-inflammatory effects of TNF inhibitors may contribute to reduced comorbidity and mortality.
The major strengths of this study include the completeness of the register of patients treated with TNF blockers in the catchment area, as well as of the comparison cohort, which can both be considered to be community-based. Furthermore, the risk of recall or information bias was minimised as the exposure information was recorded before the occurrence of the outcome, as part of a structured process, and since information regarding outcomes was achieved from an external, independent and reliable source.
One limitation of the study is the sample size, which restricts the power to study individual causes of death and to distinguish potential differences between different TNF-blocking agents.
Confounding by indication or channelling could affect this study, if patients with high risk for mortality at baseline would be less likely to be treated with TNF blockers. However, there were only minor differences in baseline comorbidities, and the effect of TNF inhibitors remained significant when controlling for comorbidities. Still, it is possible that other differences in health status, not reflected by previous hospitalisation (which is likely to underestimate comorbidity), may affect the decision to initiate anti-TNF treatment. Analyses, omitting the first 6 and 12 months of follow-up or stratifying by the date of the Food and Drug Administration warning (October 2001), on the possible danger of using TNF inhibitors in patients with severe congestive heart failure did not however substantially change point estimates. Furthermore, the assumption that patients once treated with TNF inhibitors were considered as exposed to the end of the study is conservative, which tends to move the RR towards unity.
Another limitation is the lack of information regarding exposure to traditional risk factors for CVD such as smoking, hyperlipidaemia and hypertension. Some of these possible confounders, such as smoking40 and insulin resistance,41 are clearly associated with severe RA and would thus be expected to be over-represented in the anti-TNF-treated patients, resulting in an underestimation of the protective effect of TNF blockers. It is, however, not possible to retrospectively establish whether such factors were evaluated by the treating physicians, and how it affected their decision to initiate anti-TNF treatment.
Guidelines for the use of TNF inhibitors in patients with RA were issued early by the Swedish Association for Rheumatology and have been used consistently during the study period. These guidelines recommend the use of such treatment only in patients with active disease, who have failed methotrexate and have no contraindications.
In conclusion, this investigation supports the hypothesis that inflammation and disability have a critical role in premature mortality in patients with RA and that this effect can be to some extent inhibited by treatment with TNF blockers.
REFERENCES
Footnotes
Published Online First 11 December 2006
Funding: This work was supported by grants from The Swedish Medical Research Council, King Gustaf V 80-year Foundation, The Swedish Rheumatism Association, and the Österlund and the Kock Foundations.
Competing interests: None.
Ethical approval: This study was approved by the research ethics committee at Lund University, Lund, Sweden.