Article Text
Abstract
Objectives: To develop evidence-based recommendations for the diagnosis of hand osteoarthritis (OA).
Methods: The multidisciplinary guideline development group, representing 15 European countries, generated 10 key propositions regarding diagnosis using a Delphi consensus approach. For each recommendation, research evidence was searched for systematically. Whenever possible, the sensitivity, specificity and likelihood ratio (LR) were calculated; relative risk and odds ratios were estimated for risk factors for hand OA. Quality of evidence was categorised using the European League Against Rheumatism (EULAR) hierarchy, and strength of recommendation was assessed by the EULAR visual analogue scale.
Results: Diagnostic topics included clinical manifestations, radiographic features, subgroups, differential diagnosis, laboratory tests, risk factors and comorbidities. The sensitivity, specificity and LR varied between tests depending upon the cut-off level, gold standard and controls. Overall, no single test could be used to define hand OA on its own (LR <10) but a composite of the tests greatly increased the chance of the diagnosis. The probability of a subject having hand OA was 20% when Heberden nodes alone were present, but this increased to 88% when in addition the subject was over 40 years old, had a family history of nodes and had joint space narrowing in any finger joint.
Conclusion: Ten key recommendations for diagnosis of hand OA were developed using research evidence and expert consensus. Diagnosis of hand OA should be based on assessment of a composite of features.
Statistics from Altmetric.com
Hand osteoarthritis (OA) is highly prevalent.1 ,2 It occurs commonly, though not exclusively, in the context of generalised OA,3 – 5 and can result in considerable disability.6 ,7 Although a number of criteria have been used to define hand OA (HOA),8 – 11 diagnosis presents certain difficulties due to the large number of joints involved, the broad spectrum of disease severity and possible subsets (table 1).
After developing evidence-based recommendations for management of knee OA,12 ,13 hip OA14 and HOA,15 the EULAR OA Task Force agreed that issues relating to diagnosis of HOA merit their own consideration. Therefore the following recommendations were developed using an evidence-based format involving a systematic review of research evidence and expert consensus.16
METHODS
A multidisciplinary guideline development group, comprising 21 OA experts from 15 European countries, was commissioned by the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Each participant contributed independently up to 10 propositions related to key clinical aspects in diagnosis of HOA. Consensus was reached using the Delphi technique.15 As before,15 ,17 a systematic search of the literature published between January 1945 and January 2006 was undertaken; the search for HOA15 was combined with searches for each diagnostic issue17 (see extended version of this article online at http://ard.bmj.com for full search details). The best available evidence was used to support the recommendations according to the EULAR hierarchy for diagnostic tests (table 2).17 Statistical pooling was undertaken as appropriate if there was no systematic review.18
Outcome measures
As there is no agreed gold standard for diagnosis of HOA, established methods such as radiographic changes and expert diagnosis were used as the diagnostic reference to determine the validity of a test. Validity was evaluated by sensitivity, specificity and likelihood ratio (LR) (LR = sensitivity/(1–specificity)).17 LRs above 10 or below 0.1 are considered strong evidence to respectively rule in or rule out a diagnosis in most circumstances.19 For continuous data, we used receiver operating curve (ROC).20 ROC = 1 means 100% sensitive and specific. Test reliability was assessed using kappa statistics (dichotomous data) and intra-class correlation analysis (continuous data). Relative risk (RR) and odds ratio (OR) were calculated for risk factors and comorbidities associated with HOA.15 ,21 For economic evaluations, the incremental cost-effective ratio (ICER) was presented.17 Strength of recommendation (SOR) was graded using the EULAR 0–100 mm visual analogue scale (VAS).14
Future research agenda
After the propositions for diagnosis had been searched, reviewed and discussed, each participant proposed independently 10 propositions for future research. Consensus was obtained using the Delphi technique.
RESULTS
General literature
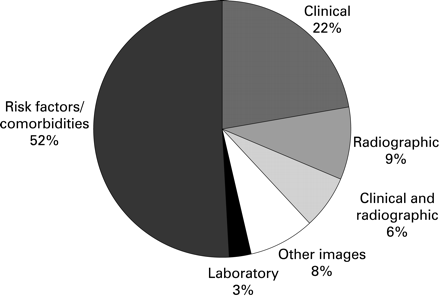
The literature search yielded 6101 hits. After deleting duplications, 2525 hits remained. Of them, only 108 studies met the inclusion criteria. Whist over half of them (52%) were studies for risk factors or comorbidities, others were studies for clinical features (22%), radiographs (9%), clinical and radiographic features (6%), other imaging (8%) (eg, ultrasound, MRI, scintigraphy) and laboratory markers (3%) (eg, erythrocyte sedimentation rate (ESR) and rheumatoid factor (RF)) (fig 1). Radiographs were the main “gold” standard used in these studies (39%). Other “gold” standards included clinical (21%), clinical and radiographic (23%) and indeterminate (17%). The majority of studies were cross-sectional (63%), followed by case control (23%), cohort (11%) and systematic review (3%).
EULAR recommendations
Of 184 propositions suggested for diagnosis, 10 were agreed after 3 anonymous Delphi rounds (table 3).
Proposition 1
Risk factors for HOA include female sex, increasing age over 40, menopausal status, family history, obesity, higher bone density, greater forearm muscle strength, joint laxity, prior hand injury and occupation or recreation-related usage.
Strength of recommendation (95% CI): 69 (54 to 84)
The gender difference for HOA has been systematically reviewed, examining 2 incidence and 14 prevalence studies. Women have a slightly greater prevalent risk of HOA than men, with relative risks of 1.54 (95% CI 0.83 to 2.86) for incidence and 1.23 (95% CI 1.11 to 1.34) for prevalence respectively.22 When female gender is used as a diagnostic criterion to differentiate HOA from other types of hand arthritis the LR is not statistically significant (LR = 0.94, 95% CI 0.80 to 1.13).8
It is rare for HOA to develop before the age of 40, but after this age the incidence increases dramatically, especially in women (fig 2).23 Age has been confirmed in many studies as one of the major risk factors for HOA23 – 28 and when a cut-off of 40 years is used it has an LR of 3.73 (95% CI 2.69 to 5.18) (fig 3)8
Certain occupations such as cotton picking29 increase the risk of HOA. This was confirmed by a systematic review of 11 case control and cross-sectional studies. The risk was dose-dependent, mainly targeting distal interphalangeal (DIP) joints and metacarpophalangeal (MCP) joints but showing differential joint distribution within the hand depending on the repetitive tasks involved.30
Sex hormones may influence the development of HOA in women. It was signalled by the gender difference dependent on age, ie, women have lower incidence of HOA before the age of 40 but higher incidence after this age than men.23 ,31 The reduction in oestrogen due to the menopause is therefore suggested. However, this is not supported by the evidence observed from the hormone replacement therapy (HRT) studies, where the use of HRT was not associated with the reduced risk of HOA.32 – 35 As these studies were observational studies, they may be confounded by other factors such as the increased bone density (a potential risk factor for HOA) due to HRT.36 – 40 Randomised controlled trials in this regard are still required.
Other well established risk factors for HOA include positive family history,8 ,41 – 44 obesity,8 ,26 ,40 ,45 – 49 and joint injury.40 High forearm extensor muscle strength has also been suggested as a risk factor, presumably by increasing damaging mechanical forces in the hand50 (table 4).
In summary, major risk factors for HOA include age over 40 years (evidence level IIa), female gender (evidence level Ib), positive family history (evidence level Ib), occupational usage (evidence level Ib), obesity (evidence level IIa) and finger joint injury (evidence level IIb). However, the diagnostic usefulness of these risk factors, singly or in combination, requires further study.
PROPOSITION 2
Typical symptoms of HOA are pain on usage and only mild morning or inactivity stiffness affecting just one or a few joints at any one time; symptoms are often intermittent and target characteristic sites (DIPJs, PIPJs, thumb base, index and MCPJs). With such typical features, a confident clinical diagnosis can be made in adults aged over 40.Strength of recommendation (95% CI): 85 (77 to 92)
Pain on usage has limited value for the diagnosis of HOA. Whilst this feature has excellent reliability (kappa 0.85 to 1.00) and specificity (0.94 to 0.99), the sensitivity is extremely low (0.01 to 0.10) and the LR ranges from 0.50 to 5.50.11 However, limited duration of localised morning or inactivity stiffness is more specific to HOA than inflammatory arthritis.51 By contrast, the presence of uncharacterised hand pain (unspecified in terms of location, relationship to usage or rest) is not specific to HOA.7 Pain in HOA is variable in severity and often varies with time6
HOA mainly targets DIP, PIP and thumb base joints.8 ,24 ,25 ,27 ,28 ,52 ,53 The prevalence of symptomatic HOA is highest with DIP, followed by thumb base, PIP and MCP joints.27 ,28 ,52 ,54 The distribution of HOA clusters by row and by ray.52 ,55 The presence of OA at one finger joint is associated with OA at other finger joints in the same row (OR 6.4, 95% CI 4.3 to 9.4 in men and 5.2, 95% CI 4.5 to 6.0 in women), and the same ray (OR = 5.3, 95% CI 2.9 to 10.0 in men and 3.3, 95% CI 2.6 to 4.2 in women) of the same hand.52 HOA also shows symmetry between hands,52 ,55 – 57 more so for radiographic joint space narrowing (JSN) than for osteophyte.57 The presence of OA at a particular finger joint strongly associates with OA in the same joint of the opposite hand (OR = 14.0, 95% CI 7.1 to 27.8 in men and 29.8, 95% CI 19.2 to 46.3 in women).52
In summary, pain on usage is not a specific clinical marker for HOA (evidence level IIb). However, HOA strongly targets DIP, PIP and thumb base joints and the shorter duration of morning or inactivity stiffness plus clustering pattern and symmetric distribution may be useful to distinguish HOA from other forms of hand arthritis (evidence level IIb).
PROPOSITION 3
Clinical hallmarks of HOA are Heberden and Bouchard nodes and/or bony enlargement with or without deformity (eg, lateral deviation of IPJs, subluxation and adduction of thumb base) affecting characteristic target joints (DIPJs, PIPJs, thumb base and index and middle MCPJs).
Strength of recommendation (95% CI): 80 (69 to 90)
Heberden nodes (HN) and Bouchard nodes (BN) associate with underlying structural changes of HOA, especially osteophyte (OR = 5.15, 95% CI 4.37 to 6.08).58 – 60 However, their sensitivity and specificity for HOA vary widely from 0.3 to 0.9 depending on the cut-off grade, gold standard and control subjects. This in part may reflect the common time lag between development of nodes and appearance of structural x ray change. Subsequently HN or BN have limited value as a single diagnostic marker with an LR ranging from 0.50 to 5.50 and a median of 1.46 (fig 3). However, nodes become more useful when taken in combination with other HOA features (fig 4). For example, the probability of a subject with HOA is 20% when HN alone are considered, but this increases to 88% when the subject is over 40 years old, has a family history of HN and has joint space narrowing in any finger joint.
In brief, HN and BN are important clinical markers for diagnosis of HOA, especially when used in combination with other features of HOA (evidence level Ib). Research evidence for the diagnostic values of other clinically-derived features and their distribution is lacking (evidence level IV).
PROPOSITION 4
Functional impairment in hand OA may be as severe as in rheumatoid arthritis. Function should be carefully assessed and monitored using validated outcome measures.
Strength of recommendation (95% CI): 57 (42 to 73)
A number of studies have examined the functional impact of HOA.6 ,28 ,61 – 66 Pain64 and radiographic changes65 associate with impaired hand function in the setting of HOA. Functional impairment due to HOA may be similar in severity to that resulting from rheumatoid arthritis67 (evidence level IIb). Indeed, for many patients with HOA functional difficulty is their main presenting complaint. However, in one study the eventual functional outcome of fully established HOA (symptom onset ⩾10 years before) was found to be relatively optimistic for nodal OA but not for erosive OA.66
A number of validated instruments are available to assess hand function. These include the Health Assessment Questionnaire (HAQ),68 the Arthritis Hand Function Test (AHFT),69 the Arthritis Impact Measurement Scale 2 (AIMS2),70 the Cochin scale,71 the Score for Assessment and quantification of Chronic Rheumatic Affections of the Hands (SACRAH),67 ,72 the Functional Index for Osteoarthritis of the Hand (FIHOA)61 and the Australian/Canadian Osteoarthritis Hand Index (AUSCAN).73 A systematic review of these instruments has been undertaken.74 There is no universal instrument and the selection from these options is guided mainly by the clinical question (evidence level Ib).
PROPOSTION 5
Patients with polyarticular HOA are at increased risk of knee OA, hip OA and OA at other common target sites (generalised OA) and should be assessed and examined accordingly.
Strength of recommendation (95% CI): 77 (62 to 92)
HOA may not only affect multiple joints within the hand, it also can occur as a component of “generalised” OA. Patients with HOA have increased risk of knee OA (OR = 3.0, 95% CI 1.2 to 7.5)75 and hip OA (OR = 3.25, 95% CI 2.19 to 4.84)76 (evidence level IIb). A recent population-based cohort study that followed 1235 subjects without hip and knee OA at baseline for over 6 years showed that the risk of developing knee OA or hip OA was two times greater (OR = 2.1, 95% CI 1.3 to 3.1) in those with HOA than in those without HOA at baseline5 (evidence level IIa).
OA of recognised target joints (DIP, PIP, carpometacarpal (CMC), knee, hip) correlates with each other which often appear in a cluster of three or more involved groups.25 The strongest associations occur for DIP and PIP, followed by PIP and CMC, CMC and knee, PIP and knee, knee and hip and DIP and knee (table 5). These data support the concept of “generalised OA” in which some individuals are at increased risk of multiple joint involvement by OA. Classification criteria for generalised versus focal OA have been proposed.4 There is clear justification to include assessment of other target joints for OA for the purpose of diagnosis and treatment planning of HOA.
PROPOSITION 6
Recognised subsets with different risk factors, associations and outcomes (requiring different assessment and management) include IPJ OA (with or without nodes), thumb base OA and erosive OA. Each may be symptomatic or asymptomatic.
Strength of recommendation (95% CI): 68 (56 to 79)
A number of studies have identified differences between erosive and non-erosive OA (see proposition 7) (evidence level IIa–IIb). Although HOA clusters by joints, population-based cross-sectional studies have confirmed that isolated thumb base OA is a common occurrence.77 Apart from the location, thumb base OA may associate with different risk factors from IPJ OA, although both may share a similar genetic risk.41 For example, hypermobility has been reported as a risk factor for thumb base OA78 but a negative risk (“protective”) factor for IPJ OA.78 ,79 Studies on functional impairment have not confirmed any clear difference between thumb base OA and IPJ OA80 (evidence level IIb), however the long-term functional outcome for erosive OA appears worse than for nodal OA.66 Further research is required to define how clearly such subsets are delineated.
PROPOSITION 7
Erosive hand OA targets IPJs and shows radiographic subchondral erosion, which may progress to marked bone and cartilage attrition, instability and bony ankylosis. Typically it has an abrupt onset, marked pain and functional impairment, inflammatory symptoms and signs (stiffness, soft tissue swelling, erythaema, paraesthesiae), mildly elevated C-reactive protein (CRP) levels and a worse outcome than non-erosive IPJ OA.
Strength of recommendation (95% CI): 87 (81 to 93)
An age and gender matched case control study has compared radiographic features of erosive OA (n = 33) and nodal OA (n = 33) using summated scores for individual OA features (JSN, osteophyte, subchondral sclerosis, subchondral cysts) at different joint sites. Erosive OA had significantly higher scores than nodal OA at DIP, PIP and thumb IP joints, but not at MCP and CMC joints, supporting the selective targeting of IP joints by erosive OA.81 This observation is supported by two cohort studies.82 ,83 In two case control studies subchondral erosion, bony collapse and ankylosis of IP joints appeared specific to erosive OA.81 ,84 In one case control study comparing hand function in patients with erosive (n = 10), nodal OA (n = 57) and normal subjects (n = 52), hand function was worse in the patients with erosive OA.66
One case control study has examined differences in capillaroscopic abnormalities between erosive OA and nodal OA. Although some statistically significant differences were found for frequency of microhaemorrhages, tortuous capillary loops and shortened loops, they did not prove very discriminatory with LRs of 2.19 (95% CI 0.62 to 7.78), 1.21 (1.85 to 1.74) and 3.29 (1.34 to 8.07) respectively.85
Serum CRP levels have been measured in a case control study examining 67 patients with erosive OA and 31 patients with non-erosive OA. CRP levels were higher in the erosive OA group and the correlations between CRP level, radiographic severity scores and number of joints involved supported CRP as an indicator of disease activity.86 No differences in serum levels of type II cartilage biomarkers (Col2-3/4C, C2C and CS846 epitope) were demonstrated between 30 patients with erosive OA and 29 patients with non-erosive OA.87
Ultrasound has been investigated as a means to differentiate erosive OA, non-erosive OA and normal joints. One case control study (n = 60) including 20 subjects per group found ultrasound to differentiate erosive OA from normal (ROC 0.75; ROC 1 means 100% sensitive and specific) and non-erosive OA from normal (ROC 0.73), but not erosive OA from non-erosive OA (ROC: not reported).88
In summary, erosive OA appears to be a specific subgroup of HOA with worse clinical and structural outcomes. It targets mainly the IP joints with structural changes that are often severe (subchondral erosion, ankylosis) and inflammation (elevated CRP) (evidence level IIa–IIb).
PROPOSITION 8
The differential diagnosis for HOA is wide. The commonest conditions to consider are psoriatic arthritis (which may target DIPJs or affect just one ray), rheumatoid arthritis (mainly targeting MCPJs, PIPJs, wrists), gout (which may superimpose on pre-existing HOA) and haemochromatosis (mainly targeting MCPJs, wrists).
Strength of recommendation (95% CI): 81 (73 to 89)
The differential diagnosis between HOA and other arthropathy may be based on clinical manifestations (eg, age, gender, onset and progression of symptoms, degree of stiffness, joints involved (fig 5), presence of HN/BN, examination findings of synovitis and/or damage), radiographic changes (fig 6) and laboratory tests. However, as for diagnosis, a single criterion on its own has limited sensitivity and specificity (⇑ figs 3 and 4). For example, although DIP joints are mainly targeted by OA they can also be involved in rheumatoid arthritis (RA),9 inflammatory symptoms and signs and elevation of CRP may occur with erosive OA and RA, radiographic changes of HOA and calcium pyrophosphate dehydrate deposition disease (CPPD) associated arthritis are extremely similar,89 and HOA may coexist with CPPD,90 ,91 gout or RA.
A composite of multiple features is more useful, such as age, female gender, joint distribution, bone swelling (not soft tissue) and radiographic changes. Laboratory tests, although non-specific, may assist in this, for example strongly positive RF is supportive of RA and elevated urate may support gout. Some individual features do have high specificity (eg non-proliferative marginal erosion for RA, urate crystals for gout).
In brief, differential diagnosis of HOA and other types of hand arthritis depends largely on the use of a composite of features (evidence level Ib). Certain features for individual diseases may be useful for specific cases (evidence level IIb).
PROPOSITION 9
Plain radiographs provide the gold standard for morphological assessment of HOA. A posteroanterior radiograph of both hands on a single film/field of view is adequate for diagnosis. Classical features are joint space narrowing, osteophyte, subchondral bone sclerosis and subchondral cyst; subchondral erosion occurs in erosive hand OA. Further imaging modalities are seldom indicated for diagnosis.
Strength of recommendation (95% CI): 87 (81 to 93)
Structural changes on plain radiographs have been used by the majority of studies as the “gold standard” for the assessment of a diagnostic test.92 – 95 The validity of radiographic change itself has been examined in two case control studies in which the clinical diagnosis was used as the “gold standard”.8 ,9 Classical radiographic features such as JSN and osteophyte are sensitive (sensitivity 0.75–1.0) but not specific (specificity 0.18–0.71), resulting in small LRs (pooled LR1.60, 95% CI 1.29 to 1.99 for JSN and 1.61, 95%CI 1.12 to 2.33 for osteophyte) (fig 3). Thus, a single feature (eg, JSN or osteophyte) is less valuable for the diagnosis than a composite of two or more features (fig 4).
The intra-reader reliability (kappa) of radiographic features for HOA ranges from 0.38 to 1.0 (kappa 0.56–1.00 for PIP joints, 0.38–0.87 for DIP joints and 0.58–0.69 for CMC-1 joints) and the inter-reader reliability (kappa) ranges from 0.52–0.92.96 The latter may be improved by reader’s experience (0.92–1.00).97 ,98 Reliability also varies according to the scale used, eg, the Verbruggen and Kellgren and Lawrence scale may have better reproducibility than global and Kallman scales.98
Other images such as scintigraphy and MRI have been investigated, especially for the early diagnosis of HOA.92 ,94 ,99 However, their values have yet to be confirmed.
In summary, the plain radiograph is the validated principle imaging technique to examine morphological changes of HOA (evidence level IIb). Diagnosis based on a single radiographic feature (eg, JSN or osteophyte) has limited value, whereas presence of multiple features, especially a composite of clinical and radiographic changes, dramatically improves diagnostic certainty (evidence level Ib). Other imaging techniques are relatively understudied and their clinical applications have yet to be determined.
PROPOSITION 10
Blood tests are not required for diagnosis of HOA but may be required to exclude coexistent disease. In a patient with HOA who has marked inflammatory symptoms and/or signs, especially involving atypical sites, blood tests should be undertaken to screen for additional inflammatory arthritides.
Strength of recommendation (95% CI): 78 (63 to 92)
Unlike RA or other forms of inflammatory arthritis, inflammatory markers are not usually elevated in HOA. It is well documented that ESR, RF (evidence level Ib) and CRP (evidence level IIb) are usually normal/negative or only mildly elevated/positive in non-erosive OA (fig 3).8 ,9 ,51 ,85 ,86 Therefore more pronounced abnormalities should lead to a search for an alternative explanation. However, as discussed, a single blood test may be unable to differentiate between erosive OA and RA, or confirm the presence of coexisting inflammatory arthropathy. It is necessary to consider other clinical and investigational features that are more characteristic and/or specific for each condition (eg, proliferative or non-proliferative marginal erosions in psoriatic and rheumatoid arthritis respectively, elevated serum uric acid and urate crystal identification on aspiration of a joint or tophus in gout).
FUTURE RESEARCH AGENDA
After three Delphi rounds, nine propositions were developed (table 6).
DISCUSSION
To our knowledge these are the first evidence-based recommendation for diagnosis of HOA. To date, the main reference cited for diagnosis of HOA has been the American College of Rheumatology (ACR) criteria for classification of HOA.8 However, the current recommendations differ from the ACR criteria in several important ways. Firstly, the primary purpose of these recommendations is to provide guidance to assist clinicians to diagnose HOA, not to classify HOA for research or clinical trial purposes. The emphasis is on possible subsets and the differential diagnosis to be considered rather than on algorithms for classification of a single entity. Secondly, these are evidence-based recommendations in which research evidence has been summarised systematically from multiple studies undertaken in different countries. Therefore they have more generalisability than recommendations based on a single study population. Thirdly, clinical expertise from many countries across Europe has been incorporated within the recommendations, and importantly, the expertise has been synthesised systematically using a Delphi exercise. Therefore, the recommendations have less parochial and personal bias.100 Finally, the strength of recommendation and confidence interval has been provided for each proposition, based on research evidence and clinical expertise. This importantly reflects the magnitude of support for each statement and the confidence (variability of opinion) from the Task Force.100 This information helps clinicians to gauge which statements have good general agreement and which are more open to personal interpretation.
The topics of the 10 generated propositions are wide ranging and include risk factors for HOA, clinical manifestations, subsets, differential diagnosis, imaging and laboratory tests. The sensitivity and specificity for each recommended marker or test has been examined and the value of each has been presented as a likelihood ratio to allow estimation of the likelihood of HOA given a positive test result. A diagnostic ladder has also been provided to show the probability of diagnosis of HOA when multiple features are considered. Clinicians may estimate the probability of HOA for any composite of the features that a patient may present on the knowledge of the likelihood ratio for each feature (fig 3). The Baye formula or Fagan nomogram may be used to estimate the probability.17 Overall we found that the diagnosis of HOA cannot be determined with confidence using a single feature and that a composite of several features is required to diagnose HOA.
There are limitations to these recommendations. Firstly, although the evidence-based method is an accepted strategy to increase the power and generalisability of research evidence, it is still open to bias since the pooled studies may carry different confounding factors. Secondly, we only focused on key issues relating to diagnosis of HOA and did not attempt a comprehensive review. Thirdly, generation of the recommendations was driven from a clinical perspective and the relevant research was examined later. Therefore we may have omitted important emerging research information. Such bias, however, should have been minimised by the general literature search and discussion undertaken prior to the Delphi exercise. Finally, the Delphi consensus approach has its own limitations. Although it is systematic it has restricted flexibility and as a result some propositions may overlap or appear repetitive or illogical. Therefore we discussed the final list of the Delphi results at our last meeting to agree on necessary changes to improve clarity. During this, however, we did not delete or introduce content but did alter some phrasing and ordering of content.
In conclusion, 10 key recommendations for diagnosis of HOA have been generated and the level of research evidence and strength of recommendation have been provided for each. We hope these recommendations will stimulate debate and increase interest in HOA and thereby lead to improved diagnosis and assessment of people with this prevalent condition.
Acknowledgments
The authors would like to thank the European League Against Rheumatism for financial support, Helen Richardson for logistical support, Jane Robertson for literature search and database development and Helen Myers and Michelle Marshall for assistance in the general search.
REFERENCES
Supplementary materials
web only appendices 68/1/8
Files in this Data Supplement:
Footnotes
Competing interests: None declared.
Funding: Financial support was received from the European League Against Rheumatism.
▸ The full version of this article is available online only at http://ard.bmj.com/content/vol68/issue1