Article Text
Abstract
Objective At 5 years' follow-up of early (<6 months) rheumatoid arthritis patients to (1) investigate whether initial combination therapy with methotrexate (MTX) and ciclosporin (CSA) (n=80) is superior to initial monotherapy with MTX (n=80) with respect to prevention of radiographic progression, (2) investigate whether the favourable clinical and radiographic response reported at 2 years in the CIMESTRA trial can be maintained and (3) identify predictors of radiographic outcome.
Methods 139 patients completed 5 years' follow-up with maintained double-blinding and a strict synovitis suppressive treatment strategy with intra-articular betamethasone injections (intra-articular glucocorticosteroid (GC)) and escalation of disease-modifying anti-rheumatic drug treatment. Disease activity, total Sharp-van der Heijde Score (TSS) of hands, wrists and forefeet were assessed at baseline and after 3, 4 and 5 years. MRI of the wrist and anti-cyclic citrullinated peptide (anti-CCP) were assessed at baseline.
Results At 5 years, TSS progression rate was <1 unit/year and 47% had not progressed radiographically since baseline. 78% were in Disease Activity Score remission, 56% in American College of Rheumatology remission and 17% withdrawn from treatment due to remission. There were no differences between initial treatment groups. MRI-bone marrow oedema, TSS and anti-CCP predicted radiographic progression at 5 years.
Conclusion Early and strict synovitis suppressive treatment with MTX and intra-articular GC lead to high remission rates and halting of erosive progression at 5 years. No additional effect of initial combination therapy with CSA was found. The results parallel those reported for tumour necrosis factor α antagonists. Baseline MRI-bone oedema, TSS and anti-CCP predicted radiographic progression.
Statistics from Altmetric.com
Rheumatoid arthritis (RA) is a chronic, inflammatory disease frequently associated with early joint destructions. Aggressive treatment with disease-modifying antirheumatic drugs (DMARDs) at the time of diagnosis improves the disease course,1,–,5 although few studies have reported long-term (>2 years) clinical and radiographical outcome.6,–,9 It has not been settled whether initial combination treatment of methotrexate (MTX) with other DMARDs is superior to MTX monotherapy.9,–,11
In the investigator-initiated randomised double-blinded placebo-controlled CIMESTRA trial, MTX monotherapy was compared with MTX and ciclosporin (CSA) combination therapy in DMARD-naïve patients with recent-onset, active RA. Intra-articular glucocorticoid injections were given on demand in both treatment groups aiming at suppression of clinical synovitis. After 2 years, American College of Rheumatology (ACR) and Disease Activity Score (DAS28) remission rates were 35–43% and 50–51%, respectively.2 Joint destructions were effectively halted, since 66–74% did not progress radiographically, and the average progression rate was <1 Sharp score unit/year.2
One of the challenges in the management of early RA is to identify poor prognosis patients with high risk of progressive joint destructions. We have previously shown that the best predictor at baseline for radiographic progression after 2 years was MRI bone marrow oedema.12
This paper presents the results of the 5-year extension of the CIMESTRA trial. The patients continued a strict synovitis suppressive treatment protocol. The aims were to investigate at 5 years whether initial combination therapy was superior to initial monotherapy regarding prevention of radiographic progression, whether the excellent radiographic and clinical results achieved after 2 years were maintained, and finally to identify baseline predictors of radiographic progression.
Patients and methods
Study design
This report presents 5-year data from the CIMESTRA trial. Details of the first 2 years have been published.1 2 Radiographic status, disease activity and adverse events were assessed annually on an intent-to-treat basis.
Patients
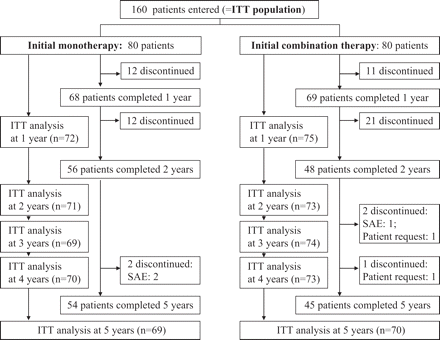
The study included 160 RA patients. Inclusion criteria were fulfilment of the ACR 1987 revised criteria for RA,13 active disease with ≥2 swollen joints and disease duration <6 months. A total of 143, 143 and 139 patients attended the visits after 3, 4 and 5 years, respectively (figure 1).
Patient disposition.
Treatment strategy
The primary strategy was early and sustained synovitis suppression by intra-articular glucocorticosteroids (GCs) and conventional DMARDs. The first 2 years of the CIMESTRA trial consisted of two treatment arms: (1) MTX plus CSA (initial combination therapy group) and (2) MTX plus placebo-CSA (initial monotherapy group). In case of swollen joints, intra-articular betamethasone was administered, and the MTX dose increased gradually from 7.5 to 20 mg/week in both treatment arms. During the first year, CSA/placebo-CSA was increased stepwise (0.5 mg/day every 4 weeks) from 2.5 to 4.0 mg/kg in patients with active disease despite maximum MTX dosage.1 From week 76 to week 104, CSA/placebo-CSA was tapered to zero, while MTX was continued. Hydroxychlorochine (HCQ) 200 mg/day was added in all patients at week 68. Oral prednisolone was not allowed during the first 2 years.
From year 3 to 5, the treatment strategy of strict clinical synovitis suppression by intra-articular GCs and conventional DMARDs was continued, and the initial treatment arm assignment remained double-blinded. Patients who did not achieve an ACR20 treatment response despite 20 mg MTX per week were switched to triple therapy (MTX, sulphasalazine (SSZ) and HCQ). If disease activity persisted, the patients were switched to tumour necrosis factor α inhibitor therapy and followed on intent-to-treat basis. Patients in ACR remission ≥12 months at the annual visit were offered gradual withdrawal from treatment (first MTX, then HCQ).
All patients received folic acid, calcium and vitamin D supplementation. Patients with a Z-score <0 in the femoral neck or lumbar spine at baseline (by dual-energy x-ray absorptiometry scan) received alendronate 10 mg/day.
Primary and secondary end points
The primary efficacy end point was radiographic progression (change in total Sharp-van der Heijde score (TSS)) at 5 years compared to baseline.14 Secondary end points included radiographic progression at 3 and 4 years, as well as clinical remission and functional disability at 3, 4 and 5 years.
Conventional radiographs of hands (posteroanterior and Norgaard15 projections), wrists (posteroanterior and lateral projections) and forefeet (anteroposterior view) were obtained. The radiographs were scored by an independent senior musculoskeletal radiologist (AV),16 blinded to all patient data, but not to chronology of the images. The estimated annual rate of progression in the TSS was calculated according to the duration of disease and the baseline TSS for each patient.17
Remission was defined according to the ACR criteria for remission in RA18 and according to the DAS28 <2.6.19 Sustained remission was defined as patients who were in ACR remission at both 3, 4 and 5 years. Functional disability was assessed annually with the Health Assessment Questionnaire (HAQ) without correction for devices.
Potential baseline predictors
Anti-CCP IgG antibodies were determined by a second-generation ELISA (Immunoscan RA kit; Euro-Diagnostica AB, Malmö, Sweden) with 25 U/ml as cut-off point.20 Serum C reactive protein (mg/l) and erythrocyte sedimentation rate (mm/h) were measured using standard laboratory methods.
Contrast-enhanced MRI of the non-dominant wrist was performed at baseline in 130 patients. At three centres, a dedicated extremity MRI unit was used, at two centres 1.0 T and 1.5 T whole-body MRI units, respectively, were used.12 MRI sequences included coronal and axial T1-weighted images before and after intravenous gadolinium-contrast injection, and a coronal short τ inversion recovery sequence.12 The MR image sets were assessed for bone erosions, synovitis and bone marrow oedema according to the Outcome Measures in Rheumatology MRI scoring system 21 by an independent, trained rheumatologist (BE), who was blinded to all patient data.
A total of 110 patients had data available on all required baseline variables and radiographs at 5 years and were included in the prediction model.
Ethical considerations
All patients gave a renewed written informed consent to participate in the trial extension. The study was approved by the national health authorities and ethics committees (Refnumber:M-1959–98), performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation 1996 revised Guidelines for Good Clinical Practice in the European Community and registered at http://www.clinicaltrials.gov (Refnumber:NCT00209859).
Statistical analysis
Analysis was by intent-to-treat and included all available data from patients who showed up for one or more of the annual follow-up visits. In addition, completers' analysis as well as analysis in which the patients on biologic treatment were excluded, were performed with similar results (data not shown). Comparisons between groups were made with Fisher's exact test (dichotomous responses) and the Mann–Whitney U test (non-dichotomous responses). Temporal changes were analysed with McNemar's test (dichotomous responses) and Wilcoxon's signed rank sum test (non-dichotomous responses).
Longitudinal data analysis of the change in TSS over time (1-2-3-4-5 years) was performed in a linear mixed-effects model with treatment arm, baseline TSS, time and the interaction between treatment arm and time as covariates. The model was tested under condition of unstructured covariance.
Potential predictors were initially tested in univariate analyses, secondarily in a linear multiple regression analyses (with ΔTSS at 5 years as dependent variable) and multiple logistic regression models (with ΔTSS >0 at 5 years (yes/no) as the dependent variable). Data are reported as the mean ± SD (variables with normal distribution); otherwise as the median IQR. The R software package22 was used for the statistical analyses, which were performed by an independent statistician.
Results
There were no statistically or clinically significant differences regarding radiographic progression, clinical outcome or other outcomes between the two treatment groups (initial monotherapy with MTX and initial combination therapy with MTX and CSA) at 3, 4 and 5 years. Therefore, we present the results of analyses for each of the two treatment groups in table 1 and figures 2 and 3, whereas the pooled data of both treatment arms in most cases are presented in the text below.
Observed radiographic changes in the two treatment groups.
Cumulative probability plots of (A) baseline MRI bone oedema score and (B) baseline anti-CCP antibodies.
Treatment, treatment responses, remission rates and radiographical outcome measures at baseline and after 3, 4 and 5 years
Patient characteristics
The median DAS28 at inclusion was 5.26 (IQR 4.43–6.02), 65% were IgM-rheumatoid factor positive, 58% had anti-cyclic citrullinated peptide (anti-CCP) antibodies and 63% had erosive disease. Figure 1 shows patient disposition.
Treatment
At 5 years, 50% of the patients were treated with MTX or MTX and HCQ, 16% received triple therapy, while 17% were on biological treatment (table 1). Seventeen per cent had withdrawn from treatment because of ≥12 months' ACR remission.
The median number of joint injections per visit was 0.55 (IQR 0.38–0.96) during the first 2 years. On the assumption that 3 mg of betamethasone is equivalent to 20 mg of prednisolone, the median intra-articular betamethasone dose during the first 2 years corresponded to 0.97 mg prednisolone per day (IQR 0.5–1.7 mg/day), mean (SD): 1.4 (1.3) mg/day. From year 3 to 5, the median cumulated glucocorticoid dose (intra-articular and oral) corresponded to 0.09 mg/day (0–0.38 mg/day) prednisolone, mean (SD): 0.60 mg/day (1.42 mg/day).
Adverse events
Three patients were excluded because of serious adverse events during follow-up (figure 1). One patient in the combination therapy group was diagnosed with disseminated colon cancer and two patients in the monotherapy group died (oesophageal carcinoma (n=1), cardiac insufficiency (n=1)). Adverse events to betamethasone was reported in a tailored questionnaire. At ≥1 visit, the following fraction of patients reported: Central adiposity: 15.6% (mild), 3.8% (moderate), 0% (severe); moon face: 15.6% (mild), 2.5% (moderate), 0.6% (severe); flushing: 22.5% (mild), 2.5% (moderate), 0% (severe); acne: 20.6% (mild), 3.8% (moderate), 0.6% (severe); psychic symptoms: 26.3% (mild), 9.4% (moderate), 0.6% (severe).
Radiographic progression
TSS at baseline and at 5 years were 5.03 (6.67) and 9.90 (12.6), respectively (mean (SD)). Changes in TSS from baseline could be calculated in 140 patients at 3 years, 133 patients at 4 years and 130 patients at 5 years.
Figure 2 and table 1 show that there were no differences between the two treatment groups regarding the progression in TSS from baseline to 3, 4 and 5 years. This was confirmed in the linear mixed-effects model, which showed that treatment group and its interaction with time were not significant, whereas time and baseline TSS were (data not shown). The annual progression rate was 0.90±1.70 (mean±SD) after 5 years. At 5 years, 72% had erosive disease, compared to 63% at baseline. Forty-seven per cent had not progressed radiographically since baseline. This was similar in the two treatment arms.
Remission
DAS28 decreased from 5.3 (2.5–8.3) (median (range)) at baseline to 1.8 (1.0–6.6) at 5 years, table 1. ACR20, 50 and 70 treatment responses were achieved in 89%, 81% and 67%, respectively, which was largely unchanged compared to year 1 and year 2 results.1 2
The proportion of patients achieving remission remained high after year 2, and not different between the treatment groups (table 1). Thus, 56% were in ACR remission, and 78% in DAS28 remission at 5 years. Twenty-seven per cent achieved sustained remission.
The proportion of patients who had no swollen joints at 5 years was 76% versus 0% at baseline (p<0.001), no tender joints (66% vs 3%, p<0.001), or no pain (less than 10 mm) (61% vs 5%, p<0.001).
Functional disability
At baseline, 86% of patients had impaired HAQ score (≥0.25), and the median HAQ score was 0.938 (IQR 0.375–1.500). At 5 years, 43% had HAQ ≥0.25, and the median HAQ score was 0.125 (0–0.625) (table 1).
Predictors of radiographic progression
The following potential baseline risk factors for radiographic progression were included in a multiple regression analysis with ΔTSS (0–5years) as dependent variable: anti-CCP (dichotomised), MRI bone marrow oedema score, MRI erosion score, MRI synovitis score, TSS and DAS28. Age and gender were forced into the model. Smoking and its interaction with anti-CCP were not included because smoking was not significant in univariate analysis.
The final multiple linear regression model showed that baseline MRI bone marrow oedema, TSS and anti-CCP were independent predictors of radiographic progression (table 2). MRI bone marrow oedema explained 23% of the variation in the progression of the TSS (Pearson's r=0.48). Figure 3A,B shows the radiographic progression in patients with or without baseline MRI bone marrow oedema and anti-CCP, respectively.
Baseline predictors of radiographic progression after 5 years
Risk estimates of radiographic progression were:
Expected ΔTSS after 5 years = 0.16 + 0.82×baseline MRI-BMO-score + 0.27×baseline-TSS + 0.82 (if anti-CCP positive).
In logistic regression analysis with radiographic progression (+/-) as the dependent variable, and the same explanatory variables as above, baseline MRI bone marrow oedema was borderline significant (OR=1.44 (95% CI: 0.95 to 2.20), p=0.09), whereas anti-CCP (OR=4.03 (1.65 to 9.82), p=0.002) and TSS (OR= 1.12 (1.03 to 1.21), p=0.006) remained in the final models.
Discussion
To our knowledge this is the first study of DMARD treatment in early RA patients with maintained double-blinding in a standardised treatment protocol during 5 years' follow-up. It shows that aggressive DMARD treatment including intra-articular betamethasone aiming at strict synovitis suppression leads to sustained excellent disease control radiographically and clinically, and that initial MTX monotherapy was as effective as initial MTX and CSA combination therapy with respect to clinical and radiographical outcome at 5 years.
The TSS increased on average by less than 1 unit per year and almost 50% of patients did not progress during 5 years. As the treatment strategy of strict synovitis suppression was applied to both treatment arms throughout the 5 years of follow-up, the isolated impact of initial combination therapy could be studied. This is in contrast to most other long-term (ie, >2 years of follow-up) studies of combination therapy in which the design was either open, or the treatment arms differed with respect to other factors, such as visit frequency or the use of concomitant prednisolone.35,–,38 The halting of erosive progression was observed in both treatment arms and therefore should be attributed to the strategy of tight control of inflammation rather than to initial mono- versus combination therapy. It has been suggested that the trials that achieve the best clinical and radiographic results are not those, which merely compare agents, but rather those which aim at remission and maintenance of tight disease control and allow discretion of the physician to adjust therapy according to quantitative findings of inadequate response during the trial.23 Our results support this statement.
In the COBRA trial, the annual Sharp progression rate in the combination group at 5 years was 5.6 units versus 8.6 units in the SSZ group.6 In the Fin-RACo study, radiographic progression was lowest in the combination therapy group, but considerably higher than in the present study, although direct comparison is difficult because radiographic damage was assessed by Larsen score.7 Since both studies compared combination therapy with SSZ monotherapy, they do not shed light on whether MTX as first line drug should be given alone or in combination. In 145 patients with early (<1 year) RA, the patients who had received MTX, SSZ or both during the first year had similar mean DAS, HAQ and radiographic scores at 5 years with open-label, free-choice follow-up treatment strategy.9 In the BeSt study, the mean progression in TSS was 6.7 in the initial combination therapy group and 11.7 in the monotherapy group at 4 years follow-up. The design does not allow us to identify whether the improved response observed in the combination therapy group should be attributed to SSZ and HCQ or to the initial high-dose prednisolone.24
In the present study, the main clinical focus at study control visits was whether or not swollen joints were present. In case of swollen joints, intra-articular betamethasone was given and MTX dosage increased. Following this strategy, at 5 years >75% were in DAS remission, >50% were in ACR remission and >25% had achieved sustained remission over 3 years. Thus, the majority of patients had no swollen joints, tender joints or pain, and the functional impairment was not different from what has been found in a normal population.25
Compared to the COBRA trial, the average DAS28 scores from year 3 to 5 were 50% lower in the CIMESTRA study (4.0 vs 2.0, respectively).6 ACR remission rates in the Fin-RACo study were half of what was achieved in the present study (22–28%).7 It is possible that the present aggressive treatment protocol, using inexpensive antirheumatic agents in a flexible dosing regime aiming at early and sustained clinical synovitis suppression have reset the inflammatory process and the radiographic progression rate effectively during the first years, resulting in a milder long-term disease course. Thus, the present results match those reported for biologic therapies in RA at an early stage of the disease.26,–,28
One in six patients had been able to withdraw from therapy altogether after ≥12 months ACR remission. Less than 20% had switched to biologic agents, although the follow-up period took place during an era of widespread use of biological treatments. The very modest need for glucocorticoids supports the efficacy of the applied treatment strategy.
Previous studies have shown that CSA combined with MTX has significant disease-modifying effects.29 30 The low-dose (2.5 mg/kg), short-duration schedule (withdrawn during the second year) applied in the present study was safe and well-tolerated.2 We therefore consider CSA a drug that may be successfully combined with MTX. The present study showed, however, that although CSA to some degree improved the clinical responses for as long as it was given, it did not at any time point influence radiographic progression.1 2
The addition of HCQ from week 76 and onwards may have increased the potency of MTX, since coadministration of MTX with HCQ has been reported to increase the bioavailability of MTX compared to MTX administered alone.31
We have previously shown that among a panel of baseline variables, MRI bone marrow oedema was the best predictor of radiographic progression after 2 years.12 At 5 years, bone marrow oedema was still the strongest independent predictor of radiographic progression, but also presence of anti-CCP antibodies and baseline Sharp score were independent predictors. Few other studies have investigated the long-term (>1 year) predictive value of MRI, and none of them included anti-CCP. A 6-year follow-up study found MRI to be a significant predictor of radiographic progression.32 In a study of 114 patients with early RA, a high baseline combined score of wrist joint MRI erosions and synovitis was reported to be the best predictor of radiographic erosive progression in the hand, wrists and feet after 10 years.33 Bone marrow oedema was not assessed, which make direct comparison with our study difficult. The predictive value of anti-CCP for radiographic progression is in accordance with the conclusions in a recent meta-analysis and with a 10-year follow-up of 238 RA patients in whom a positive anti-CCP test increased the odds of radiographic progression by 5.7.20 34
In conclusion, the present study shows that during 5 years of follow-up of early RA, aggressive treatment with MTX aiming at suppression of synovitis with intra-articular betamethasone injections on demand lead to halted radiographic progression and remission in the majority of patients. Initial treatment with MTX in combination with CSA was not superior to initial MTX monotherapy regarding long-term clinical response and radiographic progression. MRI bone marrow oedema, anti-CCP and TSS were independent predictors of radiographic progression.
Acknowledgments
The study was supported by a grant from The Danish Rheumatism Association. Novartis Healthcare Denmark A/S kindly provided the ciclosporin (Sandimmun Neoral) and placebo-ciclosporin and sponsored an independent GCP monitor. Nycomed provided methotrexate (Emthexate), folic acid (Apovit) and calcium/vitamin D (CaviD) supplementation. Schering-Plough provided betamethasone for injections (Diprospan), and MSD provided alendronate (Fosamax). The sponsors were not involved in the study set-up, data collection, analysis or interpretation, and had no influence on the publishing of data. Statisticians Morten Aagaard Petersen and Niels Steen Krogh are acknowledged for statistical advice and analysing of data.
References
Footnotes
Participants in the CIMESTRA study group are listed in the online supplementary file.
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the Ref nr. M-1959-98.
-
Provenance and peer review Not commissioned; externally peer reviewed.