-
PDF
- Split View
-
Views
-
Cite
Cite
M. M. Haara, J. P. A. Arokoski, H. Kröger, A. Kärkkäinen, P. Manninen, P. Knekt, O. Impivaara, M. Heliövaara, Association of radiological hand osteoarthritis with bone mineral mass: a population study, Rheumatology, Volume 44, Issue 12, December 2005, Pages 1549–1554, https://doi.org/10.1093/rheumatology/kei084
Close - Share Icon Share
Abstract
Objectives. A number of previous studies have reported an inverse relationship between osteoarthritis and osteoporosis. However, the association has remained controversial because osteoarthritis in hand joints seems to associate differently from osteoarthritis in weight-bearing joints with bone mineral mass. We studied osteoarthritis in distal interphalangeal (DIP) joints and osteoarthritis in the base of the thumb (CMC-1) for their cross-sectional associations with metacarpal cortical bone mineral mass, and for their prediction of calcaneal broadband ultrasound attenuation.
Methods. A population sample of 8000 Finns aged 30 yr and over was invited to a comprehensive health examination in 1978–1980; 90% complied. Hand radiographs were taken from 3568 participants to diagnose osteoarthritis in various hand joints, and to determine two indicators of cortical bone mineral mass, the combined cortical thickness (CCT) and the metacarpal index (MCI). Calcaneal broadband ultrasound attenuation was measured 20 yr later in 340 of these participants with the Sahara sonometer.
Results. In the cross-sectional setting, osteoarthritis in the DIP joints and osteoarthritis in the base of the thumb (CMC-1) were significantly associated with low CCT and low MCI. These associations were proportional to the radiological severity of osteoarthritis. In the follow-up setting, symmetrical DIP osteoarthritis adjusted for age, sex, body mass index, smoking, education, workload and MCI significantly predicted low values of broadband ultrasound attenuation.
Conclusions. Our results indicate a direct relation of both radiological DIP osteoarthritis and CMC-1 osteoarthritis with low cortical bone mineral mass, in proportion to the severity of osteoarthritis. The presence of symmetrical DIP osteoarthritis, a possible indicator of generalized osteoarthritis, suggests an increased risk of osteoporosis over time.
The association between osteoarthritis (OA) and osteoporosis (OP) remains unclear even today, 30 yr since the first results indicating an apparent inverse relationship between these common diseases was described [1]. Recent studies indicate higher bone mineral densities measured by dual-energy X-ray absorptiometry (DXA) in subjects with knee or hip OA, but it is less clear in subjects with hand OA [2]. Furthermore, it seems that the association between OA and OP may differ between localized OA and primary generalized OA [3].
There are a number of possible mechanisms behind the association between OA and OP. One suggested mechanism is that OA might primarily be a disease of subchondral bone; stiff bone with high bone mineral mass may increase the mechanical stress on cartilage [4–7]. On the other hand, it has been postulated that osteoporotic bone might absorb load more efficiently than normal bone, thus transmitting less stress to the overlying articular cartilage [8]. Moreover, the two diseases have common confounders, such as weight and lifestyle risk factors [9]. However, in OA it is still unknown whether changes in the cartilage precede those in bones or vice versa [7, 10, 11].
While there are numerous studies on the association between OA at various sites and bone mineral mass measured by means of several techniques [5], only a few have evaluated the changes in bone mineral mass longitudinally in subjects with radiographic OA [12–15]. Furthermore, few studies have concentrated on the associations between different grades of OA and bone mineral mass [16]. Such studies would help to clarify the order of onset and association of OA and OP (to clarify which of the two comes first,) and how the severity of OA and bone mineral mass are related to each other.
Therefore, the aim of this study was to examine different grades of OA in distal interphalangeal joints (DIP) and the thumb carpometacarpal joint (CMC-1) for their cross-sectional association with metacarpal cortical bone mineral mass, and for their prediction of calcaneal broadband ultrasound attenuation (BUA) during up to 20 yr of follow-up.
Method
Population
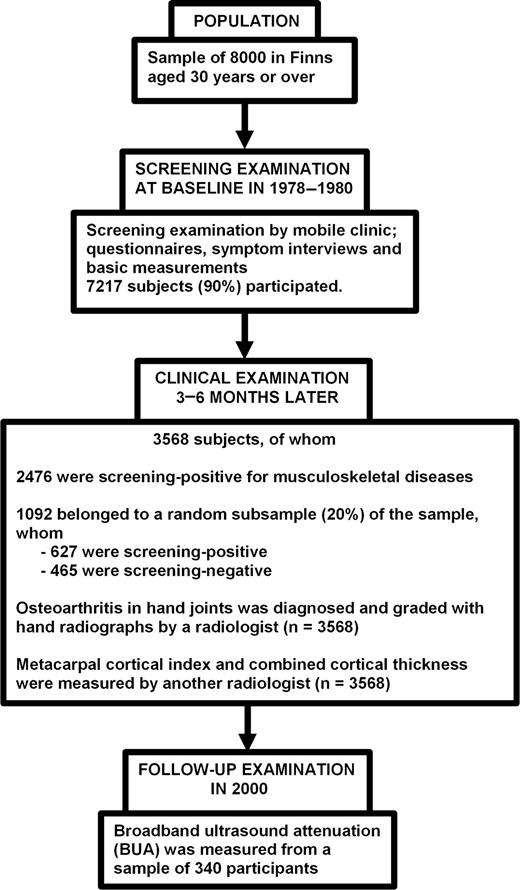
The study population was a stratified two-stage cluster sample drawn from the population register to represent Finnish adults aged 30 yr or over [17]. In the first stage, 40 representative areas were selected. In the second stage, a systematic sample of inhabitants was drawn from each area. The sample consisted of 8000 persons (3637 men and 4363 women) of whom 7217 (90%) participated in the survey in 1978–80 (Fig. 1).
The flow of operations during the Mini-Finland Health Survey and follow-up up to the year 2000.
This study precedes current legislation on medical research. Thus, participants were fully informed about it, they attended it on a voluntary basis and the use of the information for medical research was explained to them.
Baseline examination
Details of the design and implementation of the Mini-Finland Health Survey have been reported elsewhere [17]. In brief, all participants were interviewed at home and asked to fill in a basic questionnaire before attending a screening examination. The interview and questionnaire elicited essential information on health habits and previously diagnosed diseases. The screening phase comprised measurements and tests to identify subjects with possible cardiovascular, respiratory or musculoskeletal diseases. Those with findings suggestive of musculoskeletal diseases and a 20% random sample of the participants were asked to attend a clinical phase (n = 3568) (Fig. 1). Characteristics of the subjects included in the sample, such as age, sex, body mass index (BMI), smoking status, history of workload and educational level, have been described in detail elsewhere [18]. There were 1549 men and 2019 women whose age range was 30–95 (mean 54.4; s.d. 13.7) yr.
Definition of determinants
A basic questionnaire was used to elicit information on the subjects present and previous occupations involving exposure to (i) lifting or carrying heavy objects, (ii) stooped, twisted or otherwise awkward work postures, (iii) vibration of the whole body or the use of vibrating equipment, (iv) continuously repeated series of movements, and (v) work paced by a machine. Only exposures related to the present or to the most recent occupation were taken into account. These exposures were recorded as dichotomies (no = 0, yes = 1), and their total number was designated as the sum index of physical stress at work [19]. Leisure time physical activity was categorized into three groups: low, moderate and high activity. Standing height and weight were measured at the screening examination, and BMI (weight/height2, kg/m2) was used as a measure of relative weight. Smoking history was obtained in a standard interview and categorized as follows: never smoked; ex-smoker; current smoker. The level of education was divided into three categories on the basis of years of education.
Diagnosis and grading of hand osteoarthritis
Posteroanterior hand radiographs of both hands were taken from 3568 persons who met at least one of the screening criteria for musculoskeletal diseases, or belonged to the random 20% sample (Fig. 1). Hand radiographs were read mainly to diagnose OA [20]. Standard criteria [21, 22] were used for assessing joint space narrowing and osteophytes. The readings of X-rays were carried out by a radiologist without any information on the clinical findings or on metacarpal measurements assessed by another radiologist [20]. Each joint of both hands was graded individually, and classified into the following grades: 0 = no OA, 1 = doubtful OA, 2 = minimal OA, 3 = moderate OA, 4 = severe OA. OA was considered to be present if the grade was 2 or more. The reliability of the readings was estimated by measuring intra-observer and interobserver agreement, using the correlation coefficient κ as an indicator of agreement [20]. The reader classified 84.9% of the joints into the same grade in the first and the second readings; κ was 0.71. Two readers classified 73.5% of the joints into the same grade; κ was 0.53. In classifying the examinees as OA present and OA absent, the intra-observer agreement was excellent; κ was 0.89 [20]. Both interobserver and intra-observer agreements were highest in DIP joints.
Three different forms of hand OA types were defined for the present study: OA of Kellgren grade 2–4 in at least two DIP joints symmetrically (symmetrical DIP OA) [18], OA of Kellgren grade 2–4 in one or more DIP joints non-symmetrically (non-symmetrical DIP OA), and OA of Kellgren grade 2–4 in the thumb CMC-1 joint (CMC-1 OA).
Measurements of metacarpal cortical bone mass at baseline
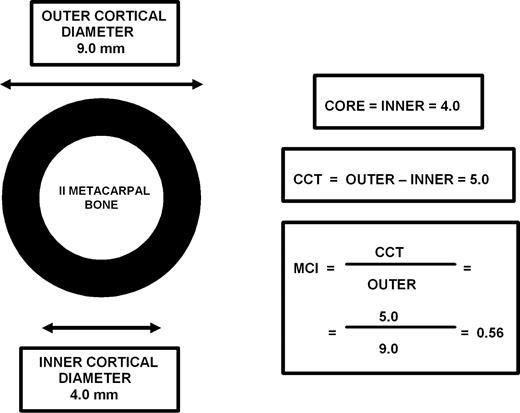
The indicators of cortical bone mineral mass combined cortical thickness (CCT) and metacarpal index (MCI) and were determined during the baseline examination in the period 1978–1980 from hand radiographs by a radiologist, other than the one who diagnosed and graded hand OA (Fig. 2). The measurements of the outer and inner cortical diameter were made at the midpoint of the second metacarpal bone of the right hand (n = 3568), with a digital calliper to the nearest 0.1 mm (Fig. 2) [20]. The coefficients of intra-observer reliability were 0.91 for both the outer and inner cortical diameter [20]. CCT was calculated as the difference between the outer and inner cortical diameters (Fig. 2.) [23]. MCI was further calculated by dividing the value of CCT by the outer cortical diameter (Fig. 2.) [20, 24].
Assessment of metacarpal core thickness (CORE), combined cortical thickness (CCT) and metacarpal index (MCI) from the midpoint of the second metacarpal bone.
Broadband ultrasound attenuation measurements at follow-up
In 2000, 340 of the participants (n = 3568) who had undergone clinical examination including radiography of hands to diagnose OA and to determine cortical measurements, were examined with a Sahara sonometer (Hologic, Waltham, MA, USA) to determine BUA (Fig. 1) [25]. The mean follow-up time was 21 yr. The sample consisted of 131 men and 209 women aged 30–71 yr at baseline and living in or around the five largest cities (Helsinki, Turku, Tampere, Oulu and Kuopio) in Finland. BUA (dB/MHz) was measured by trained nurses from the right calcaneus with a Sahara sonometer.
To evaluate the reliability and validity of field examinations proper, 42 men and 88 women aged 42–92 yr were invited to the Department of Physical and Rehabilitation Medicine Kuopio University Hospital for a comprehensive re-examination; 81% complied. Intra-observer variation was assessed on the basis of four measurements at both calcaneuses in nine men and 14 women. The intra-class correlation coefficients of BUA for the right and left calcaneus were 0.92 and 0.89, respectively. The intraclass correlation coefficient for comparisons of the right and left calcaneal measurements was 0.80. Validity was investigated by comparing the BUA measurements with DXA (Lunar Expert, Lunar Radiation Corporation, Madison, WI, USA) values of the spine (L2–L4) with femur neck bone mineral density (BMD) (g/cm2) and with the PIXI (Peripheral Instantaneous X-ray Imager, General Electric Company, Fairfield, CT, USA) value of calcaneal BMD (g/cm2) for 130 persons. Pearson's correlation coefficients for BUA were r = 0.48 with spine (L2–L4) BMD, r = 0.49 with femoral neck BMD, and r = 0.72 with calcaneal PIXI measurements.
Statistical analysis
SAS software (SAS Institute, Cary, NC, USA) was used for statistical analysis. The adjusted mean values of MCI, CCT and BUA in categories of OA, as well as multiple partial correlations of OA and other potential determinants with MCI, CCT and BUA, were estimated using the general linear model [26]. Age, sex, BMI, smoking, history of workload and educational level were entered into the model as potential confounders [18, 27–29].
Results
Radiological hand OA and metacarpal cortical bone mineral mass
In the cross-sectional setting, high age, smoking and low BMI were significantly associated with low MCI. The partial correlation coefficients and corresponding P-values were: r = −0.52, P = 0.0001 for age; r = 0.06, P = 0.0007 for smoking and r = 0.10, P = 0.0001 for BMI. These factors associated similarly with CCT: r = −0.52, P = 0.0001 for age; r = 0.07, P = 0.0004 for smoking and r = 0.14, P = 0.0001 for BMI. Female sex associated significantly with CCT (r = 0.39, P = 0.0001), but for MCI no difference was found between men and women (r = 0.009, P = 0.58). There were no significant cross-sectional associations between MCI or CCT and workload, education or leisure time physical activity (data not shown).
When adjusted for age, sex, BMI, smoking, education and workload, non-symmetrical DIP OA, symmetrical DIP OA and thumb CMC-1 OA were significantly associated with low MCI and low CCT (Table 1).
General linear model of metacarpal index (MCI), thickness of the metacarpal core and combined cortical thickness (CCT) by radiological OA of Kellgren grades 2 or 3–4 in DIP joints symmetrically and non-symmetrically (DIP OA), and by radiological OA of Kellgren grades 2 or 3–4 in thumb carpometacarpal joint (CMC-1 OA)
| Type of OA . | n . | Adjusted mean of MCI . | r . | Adjusted mean of core thickness . | r . | Adjusted mean of CCT . | r . |
|---|---|---|---|---|---|---|---|
| DIP OA | |||||||
| Not present | 2164 | 0.58 | 0.10 | 3.87 | 0.06 | 5.20 | 0.10 |
| Non-symmetrical grade 2 | 696 | 0.57 | 3.93 | 5.13 | |||
| Non-symmetrical grade 3–4 | 135 | 0.56 | 3.99 | 4.99 | |||
| Symmetrical grade 2 | 446 | 0.55 | 4.12 | 5.02 | |||
| Symmetrical grade 3–4 | 127 | 0.54 | 4.10 | 4.80 | |||
| P for heterogeneity | <0.0001 | 0.0007 | <0.0001 | ||||
| CMC-1 OA | |||||||
| Not present | 3166 | 0.57 | 0.07 | 3.90 | −0.54 | 5.16 | 0.09 |
| Kellgren grade 2 | 282 | 0.56 | 4.03 | 5.12 | |||
| Kellgren grade 3–4 | 120 | 0.54 | 4.16 | 4.79 | |||
| P for heterogeneity | <0.0001 | 0.006 | <0.0001 |
| Type of OA . | n . | Adjusted mean of MCI . | r . | Adjusted mean of core thickness . | r . | Adjusted mean of CCT . | r . |
|---|---|---|---|---|---|---|---|
| DIP OA | |||||||
| Not present | 2164 | 0.58 | 0.10 | 3.87 | 0.06 | 5.20 | 0.10 |
| Non-symmetrical grade 2 | 696 | 0.57 | 3.93 | 5.13 | |||
| Non-symmetrical grade 3–4 | 135 | 0.56 | 3.99 | 4.99 | |||
| Symmetrical grade 2 | 446 | 0.55 | 4.12 | 5.02 | |||
| Symmetrical grade 3–4 | 127 | 0.54 | 4.10 | 4.80 | |||
| P for heterogeneity | <0.0001 | 0.0007 | <0.0001 | ||||
| CMC-1 OA | |||||||
| Not present | 3166 | 0.57 | 0.07 | 3.90 | −0.54 | 5.16 | 0.09 |
| Kellgren grade 2 | 282 | 0.56 | 4.03 | 5.12 | |||
| Kellgren grade 3–4 | 120 | 0.54 | 4.16 | 4.79 | |||
| P for heterogeneity | <0.0001 | 0.006 | <0.0001 |
Data are adjusted for age, sex, BMI, smoking status, history of workload, and educational level (n = 3568).
n = number of subjects.
r = partial correlation coefficient.
General linear model of metacarpal index (MCI), thickness of the metacarpal core and combined cortical thickness (CCT) by radiological OA of Kellgren grades 2 or 3–4 in DIP joints symmetrically and non-symmetrically (DIP OA), and by radiological OA of Kellgren grades 2 or 3–4 in thumb carpometacarpal joint (CMC-1 OA)
| Type of OA . | n . | Adjusted mean of MCI . | r . | Adjusted mean of core thickness . | r . | Adjusted mean of CCT . | r . |
|---|---|---|---|---|---|---|---|
| DIP OA | |||||||
| Not present | 2164 | 0.58 | 0.10 | 3.87 | 0.06 | 5.20 | 0.10 |
| Non-symmetrical grade 2 | 696 | 0.57 | 3.93 | 5.13 | |||
| Non-symmetrical grade 3–4 | 135 | 0.56 | 3.99 | 4.99 | |||
| Symmetrical grade 2 | 446 | 0.55 | 4.12 | 5.02 | |||
| Symmetrical grade 3–4 | 127 | 0.54 | 4.10 | 4.80 | |||
| P for heterogeneity | <0.0001 | 0.0007 | <0.0001 | ||||
| CMC-1 OA | |||||||
| Not present | 3166 | 0.57 | 0.07 | 3.90 | −0.54 | 5.16 | 0.09 |
| Kellgren grade 2 | 282 | 0.56 | 4.03 | 5.12 | |||
| Kellgren grade 3–4 | 120 | 0.54 | 4.16 | 4.79 | |||
| P for heterogeneity | <0.0001 | 0.006 | <0.0001 |
| Type of OA . | n . | Adjusted mean of MCI . | r . | Adjusted mean of core thickness . | r . | Adjusted mean of CCT . | r . |
|---|---|---|---|---|---|---|---|
| DIP OA | |||||||
| Not present | 2164 | 0.58 | 0.10 | 3.87 | 0.06 | 5.20 | 0.10 |
| Non-symmetrical grade 2 | 696 | 0.57 | 3.93 | 5.13 | |||
| Non-symmetrical grade 3–4 | 135 | 0.56 | 3.99 | 4.99 | |||
| Symmetrical grade 2 | 446 | 0.55 | 4.12 | 5.02 | |||
| Symmetrical grade 3–4 | 127 | 0.54 | 4.10 | 4.80 | |||
| P for heterogeneity | <0.0001 | 0.0007 | <0.0001 | ||||
| CMC-1 OA | |||||||
| Not present | 3166 | 0.57 | 0.07 | 3.90 | −0.54 | 5.16 | 0.09 |
| Kellgren grade 2 | 282 | 0.56 | 4.03 | 5.12 | |||
| Kellgren grade 3–4 | 120 | 0.54 | 4.16 | 4.79 | |||
| P for heterogeneity | <0.0001 | 0.006 | <0.0001 |
Data are adjusted for age, sex, BMI, smoking status, history of workload, and educational level (n = 3568).
n = number of subjects.
r = partial correlation coefficient.
Radiological hand OA and BUA
In the longitudinal setting, female sex, high age and low BMI at baseline significantly predicted low calcaneal BUA values at the follow-up examination (Table 2). Workload, smoking, education or leisure-time physical activity at baseline did not predict BUA at follow-up (data not shown).
General linear model of calcaneum broadband ultrasound attenuation (BUA) by baseline age, sex, BMI, symmetrical DIP OA, MCI, smoking status (n = 340), adjusted independently of each other
| Determinant . | Number of subjects . | Adjusted mean of BUA . | r . | P . |
|---|---|---|---|---|
| Age | 340 | −0.17 | 0.001 | |
| MCI | 340 | 0.09 | 0.12 | |
| Body mass index (kg/m2) | 340 | 0.15 | 0.006 | |
| Sex | 0.34 | <0.0001 | ||
| Male | 131 | 84.9 | ||
| Female | 209 | 70.2 | ||
| Smoking status | 0.09 | 0.19 | ||
| Never smoked | 175 | 77.7 | ||
| Ex-smoker | 90 | 75.7 | ||
| Current smoker | 75 | 72.6 | ||
| Symmetrical DIP OA | 0.14 | 0.008 | ||
| No | 327 | 76.4 | ||
| Yes | 13 | 63.0 |
| Determinant . | Number of subjects . | Adjusted mean of BUA . | r . | P . |
|---|---|---|---|---|
| Age | 340 | −0.17 | 0.001 | |
| MCI | 340 | 0.09 | 0.12 | |
| Body mass index (kg/m2) | 340 | 0.15 | 0.006 | |
| Sex | 0.34 | <0.0001 | ||
| Male | 131 | 84.9 | ||
| Female | 209 | 70.2 | ||
| Smoking status | 0.09 | 0.19 | ||
| Never smoked | 175 | 77.7 | ||
| Ex-smoker | 90 | 75.7 | ||
| Current smoker | 75 | 72.6 | ||
| Symmetrical DIP OA | 0.14 | 0.008 | ||
| No | 327 | 76.4 | ||
| Yes | 13 | 63.0 |
r = partial correlation coefficient.
General linear model of calcaneum broadband ultrasound attenuation (BUA) by baseline age, sex, BMI, symmetrical DIP OA, MCI, smoking status (n = 340), adjusted independently of each other
| Determinant . | Number of subjects . | Adjusted mean of BUA . | r . | P . |
|---|---|---|---|---|
| Age | 340 | −0.17 | 0.001 | |
| MCI | 340 | 0.09 | 0.12 | |
| Body mass index (kg/m2) | 340 | 0.15 | 0.006 | |
| Sex | 0.34 | <0.0001 | ||
| Male | 131 | 84.9 | ||
| Female | 209 | 70.2 | ||
| Smoking status | 0.09 | 0.19 | ||
| Never smoked | 175 | 77.7 | ||
| Ex-smoker | 90 | 75.7 | ||
| Current smoker | 75 | 72.6 | ||
| Symmetrical DIP OA | 0.14 | 0.008 | ||
| No | 327 | 76.4 | ||
| Yes | 13 | 63.0 |
| Determinant . | Number of subjects . | Adjusted mean of BUA . | r . | P . |
|---|---|---|---|---|
| Age | 340 | −0.17 | 0.001 | |
| MCI | 340 | 0.09 | 0.12 | |
| Body mass index (kg/m2) | 340 | 0.15 | 0.006 | |
| Sex | 0.34 | <0.0001 | ||
| Male | 131 | 84.9 | ||
| Female | 209 | 70.2 | ||
| Smoking status | 0.09 | 0.19 | ||
| Never smoked | 175 | 77.7 | ||
| Ex-smoker | 90 | 75.7 | ||
| Current smoker | 75 | 72.6 | ||
| Symmetrical DIP OA | 0.14 | 0.008 | ||
| No | 327 | 76.4 | ||
| Yes | 13 | 63.0 |
r = partial correlation coefficient.
Symmetrical DIP OA at baseline significantly predicted low values of BUA 20 yr later (Table 2). This finding remained significant even when adjusted for age, sex, BMI, smoking and MCI. However, the proportion of the variance in BUA that could be explained by the symmetrical DIP OA was small (r2 = 0.02). Thumb CMC-1 OA and non-symmetrical DIP OA, on the other hand, did not significantly predict BUA values at follow-up (data not shown).
Metacarpal cortical bone mineral mass and BUA
In the longitudinal setting, when adjusted for age and sex only, low MCI significantly predicted low BUA values 20 yr later (r = 0.16; P = 0.0001). According to the complete model, however, the association was not statistically significant (Table 2). There was an association between baseline CCT and BUA at follow-up after adjustment for age and sex (r = 0.11; P = 0.04), but after further adjustment for BMI and smoking, the association was not statistically significant (r = 0.10; P = 0.08).
Radiological hand OA, MCI and BUA in the initial and follow-up cohorts
Among all the 899 subjects who were re-examined, age- and sex-adjusted BUA values at the follow-up examination in those who had and who had not participated in the hand X-ray examination were 77.4 and 75.5, respectively (P = 0.15).
Among those who had undergone the hand X-ray examination 20 yr earlier, baseline MCI (odds ratio 1.11, 95% confidence interval 0.98–1.26), CMC-1 OA (0.81, 0.47–1.42) or non-symmetrical DIP OA (0.76, 0.49–1.17) did not significantly predict subsequent selection into the re-examination. However, symmetrical DIP OA predicted decreased likelihood of re-examination (0.45, 0.25–0.81).
Discussion
Our results showed a positive association between radiological hand OA and low metacarpal cortical bone mineral mass in proportion to the severity of hand OA. Symmetrical DIP OA in hand joints significantly predicted low values of calcaneal BUA 20 yr later, indicating an increased risk of OP over time.
We used both metacarpal cortical measurements and calcaneal ultrasound to estimate bone mineral mass. Recent evidence suggests that MCI measured from hand radiographs might be successfully used for assessing bone mineral mass and quality [30, 31]. Furthermore, it is suggested that metacarpal morphometry can show the changes in subchondral bone, which has been proved to be an important structure in the aetiopathogenesis of OA [4–7]. Furthermore, in the present study there was a cross-sectional association between both MCI and CCT and high age, low BMI and smoking, which are known risk factors for low bone mineral mass [28, 29]. Therefore, we suggest that both MCI and CCT are indicators of cortical bone mineral mass.
On the other hand, calcaneal ultrasound measurement and its output variable, BUA, have been proved to be a good estimate of bone mineral mass and fracture risk [32–34]. These results are supported by our present study, where BUA correlated with BMD values measured by DXA and PIXI. Therefore, it is evident that the calcaneal BUA values are associated with total bone mineral mass.
However, the results of bone densitometry strongly depend on the method used and the anatomical site measured. DXA has been regarded as the gold standard for the diagnosis of OP. A major limitation of our study was that MCI from plain radiographs was used at baseline, whereas BUA was measured by calcaneal ultrasound at the follow-up examination. Change in bone mineral mass could therefore not be computed precisely. Optimally, both hand radiography and DXA should have been carried out both at the baseline and follow-up surveys. Nevertheless, symmetrical DIP OA is likely to predict bone loss at follow-up, because the association was strong and not confounded by baseline MCI.
Since only a minority of the initial sample was re-examined in the current study, selection bias is possible. However, MCI, CMC-1 OA, non-symmetrical DIP OA or baseline screening for musculoskeletal conditions did not suggest any association with subsequent participation in the re-examination survey. Unexpectedly, symmetrical DIP OA significantly predicted decreased the likelihood of re-examination. If symmetrical DIP OA were to predict an increased likelihood of re-examination, its association with low BUA 20 yr later could be explained by selection. Since the reverse proved true in the present study, the question about influences of selection remains open. In general, however, our results suggesting an increased risk of OP in the presence of symmetrical DIP OA may not have resulted from systematic selection.
Somewhat unexpectedly, MCI or CCT did not predict BUA. There was a significant association between both MCI and CCT and BUA as adjusted only for age and sex. The significance, however, was lost after further adjustment for other determinants, probably because of reduced statistical power due to the small number of subjects with BUA measured at the follow-up. Also, measurements of MCI and CTT had been made 20 yr before the measurements of BUA. During the long follow-up time a number of biological factors have possibly influenced the bone mineral mass. On the other hand, only moderate correlations were observed between BUA and femoral neck and spine (L2–L4) BMD values, indicating that perhaps it was not so surprising after all that MCI or CCT was not predictive of BUA.
In previous studies, it has been suggested that OA and OP have a positive association in hand joints [12, 35]. Schneider and colleagues [35] found in their cross-sectional study that women with clinically diagnosed hand OA had significantly lower femoral BMD. On the other hand, in their 23-yr follow-up study of radiological hand OA and metacarpal bone loss, Sowers and colleagues [36] concluded that women who later developed hand OA were more likely to have a higher baseline bone mass than women who did not develop OA. These women also had a greater likelihood of bone loss over time. In their recent study, Hochberg and colleagues [12] found that women with radiographic hand OA had a significantly greater adjusted rate of bone loss at the radius than women with normal hand radiographs. Like others [12, 35, 36], we found an association between hand joint OA and decreased bone mineral mass. In the cross-sectional setting, there was a clear association between both DIP joint OA and CMC-1 joint OA and low MCI and CCT. These results suggest that OA and low cortical bone mineral mass are linked in hand joints.
Interestingly, we found that the association between hand OA and cortical bone mineral mass varies between different sites and grades of hand OA. Symmetrical DIP OA and CMC-1 OA had a stronger association with low bone mineral mass determined by MCI and CCT, compared with non-symmetrical DIP OA. The associations between all forms of hand OA and low cortical bone mineral mass proved to be proportional to the severity of hand OA. To our knowledge, such a result has not been reported from previous studies concerning hand OA and bone mineral mass [12, 35–36]. However, Bruno and colleagues [16] noticed that hips with OA of Kellgren–Lawrence scores of 1 or 2 had increased BMD throughout the proximal femur, but BMD declined as OA progressed. It has also been postulated that women with radiological knee osteophytes (moderate OA) had higher femoral BMD than those with no osteophytes [37], but joint space narrowing (severe OA) had less influence on BMD. The assumed inverse association between moderate OA in weight-bearing joints and high bone mineral mass might be due to mechanical factors leading to osteophyte formation in the joint. When OA progresses towards joint space narrowing in severe OA, disability and pain tend to make patient avoid physical activity, which leads to secondary OP. However, in hand joints the decrease in bone mineral mass with increasing severity of hand OA may suggest that hand joints are less resistant to mechanical loading during the progress of OA and that hand osteophytosis seems to have a less specific association with bone mineral mass. Therefore, we suggest that genetic and metabolic factors may explain the direct relation of hand OA with low cortical bone mineral mass.
During the follow-up, however, only symmetrical DIP OA at baseline predicted lower values of BUA. Thus, patients with symmetrical OA in DIP joints apparently lose bone at different rates than those with no symmetrical DIP OA. On the other hand, this finding suggests that there is an association between symmetrical DIP OA and OP. This is an interesting result, because previous studies have indicated that genetic factors may have a role in the aetiology of symmetrical hand OA [38, 39]. Furthermore, symmetrical DIP joint OA seems to associate more often with genetically determined generalized OA than OA in other joints [40, 41]. Generalized OA is a form of OA, but the classification is controversial [41]. According to Kellgren and Moore [42], generalized OA is present if at least three joints or a group of joints have been affected. Over 50 yr ago, Kellgren postulated that Heberden's nodes in hand joints are associated with generalized OA [42]. Recently, however, radiological DIP osteophytes were considered a better marker of knee and multiple joint OA than Heberden's nodes [41]. In our previous study, there was a strong association between symmetrical DIP OA and Heberden's nodes [18]. Since symmetrical DIP joint OA is associated with generalized OA, patients with generalized OA might lose bone more rapidly than those without this form of OA. This result needs confirmation from other studies. Thus far, it is premature to add symmetrical DIP OA to the list of established risk factors for OA. Nevertheless, already at this point we suggest that, in clinical practice, patients with symmetrical DIP joint OA should be considered to be at increased risk of OP.
In conclusion, our results indicate a direct cross-sectional relation between both radiological DIP OA and CMC-1 OA and low cortical bone mineral mass in proportion to the severity of OA. Furthermore, symmetrical DIP OA predicts low bone mineral mass estimated by BUA at 20 yr of follow-up. Patients with symmetrical DIP OA, an indicator of generalized OA, might thus be considered candidates for evaluation of a risk of OP and fracture in further studies.

This study was supported partly by EVO funding of Kuopio University Hospital, the Norther-Savo Cultural Foundation and the Duodecim Foundation, Finland. MSD-Finland supported the execution of the follow-up survey. The authors thank Marianna Sunnari and Jacqueline Välimäki from the University of Turku, Finland, for checking the language in this article.
In support of the research or preparation of this manuscript, M.M.H. received grants or outside funding from the EVO funding of Kuopio University Hospital, the Norther-Savo Cultural Foundation and the Duodecim Foundation, Finland. None of the authors received payments or other benefits, nor a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or non- profit organizations with which the authors are affiliated or associated. The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
References
Foss MV, Byers PD. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur.
Sambrook P, Naganathan V. What is the relationship between osteoarthritis and osteoporosis?
Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography.
Dequeker J, Mokassa L, Aerssens J. Bone density and osteoarthritis.
Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading.
Dequeker J. Inverse relationship of interface between osteoporosis and osteoarthritis.
Naganathan V, Zochling J, March L, Sambrook PN. Peak bone mass is increased in the hip in daughters of women with osteoarthritis.
Burr DB. The importance of subchondral bone in osteoarthrosis.
Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation.
Hochberg MC, Lethbridge-Cejku M, Tobin JD. Bone mineral density and osteoarthritis: data from the Baltimore Longitudinal Study of Aging.
Arden NK, Nevitt MC, Lane NE et al. Osteoarthritis and risk of falls, rates of bone loss, and osteoporotic fractures. Study of Osteoporotic Fractures Research Group.
Sowers MF, Hochberg M, Crabbe JP, Muhich A, Crutchfield M, Updike S. Association of bone mineral density and sex hormone levels with osteoarthritis of the hand and knee in premenopausal women.
Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study.
Bruno RJ, Sauer PA, Rosenberg AG, Block J, Sumner DR. The pattern of bone mineral density in the proximal femur and radiographic signs of early joint degeneration.
Aromaa A, Heliövaara M, Impivaara O et al.
Haara MM, Manninen P, Kroger H et al. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality.
Makela M, Heliovaara M, Sievers K, Knekt P, Maatela J, Aromaa A. Musculoskeletal disorders as determinants of disability in Finns aged 30 years or more.
Kärkkäinen A.
Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films.
Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis.
Meema HE. The occurrence of cortical bone atrophy in old age in osteoporosis.
Barnett E, Nordin BEC. The radiological diagnosis of osteoporosis: a new approach.
Aromaa A, Koskinen S, eds.
Hannan MT, Anderson JJ, Pincus T, Felson DT. Educational attainment and osteoarthritis: differential associations with radiographic changes and symptom reporting.
Kröger H, Tuppurainen M, Honkanen R, Alhava E, Saarikoski S. Bone mineral density and risk factors for osteoporosis—a population-based study of 1600 perimenopausal women.
Hyldstrup L, Nielsen SP. Metacarpal index by digital X-ray radiogrammetry: normative reference values and comparison with dual X-ray absorptiometry.
Huopio J, Kröger H, Honkanen R, Jurvelin J, Saarikoski S, Alhava E. Calcaneal ultrasound predicts early postmenopausal fractures as well as axial BMD. A prospective study of 422 women.
Frost ML, Blake GM, Fogelman I. Quantitative ultrasound and bone mineral density is equally strongly associated with risk factors for osteoporosis.
Khaw K-T, Reeve J, Luben R et al. Prediction of total and hip fracture risk in men and women by quantitative ultrasound of calcaneus: Epic-Norfolk prospective population study.
Schneider DL, Barrett-Connor E, Morton DJ, Weisman M. Bone mineral density and clinical hand osteoarthritis in elderly men and women: the Rancho Bernardo study.
Sowers M, Zobel D, Weissfeld L, Hawthorne VM, Carman W. Progression of osteoarthritis of the hand and metacarpal bone loss. A twenty-year follow-up of incident cases.
Hannan MT, Anderson JJ, Zhang Y, Levy D, Felson DT. Bone mineral density and knee osteoarthritis in elderly men and women. The Framingham Study.
Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics.
Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study.
Hirsch R, Lethbridge-Cejku M, Scott WW et al. Association of hand and knee osteoarthritis: evidence for a polyarticular disease subset.
Cicuttini FM, Baker J, Hart DJ, Spector TD. Relation between Heberden's nodes and distal interphalangeal joint osteophytes and their role as markers of generalised disease.
Author notes
Bone and Cartilage Research Unit (BCRU) and Department of General Practice and Public Health, University of Kuopio, 1Department of Physical and Rehabilitation Medicine and 2Department of Surgery/Orthopaedics and Traumatology, Kuopio University Hospital, 3National Public Health Institute, Helsinki and Turku and 4Finnish Institute of Occupational Health, Kuopio, Finland.




Comments