-
PDF
- Split View
-
Views
-
Cite
Cite
Robert M. Perkins, Joel C. Reynolds, Tejinder S. Ahuja, Thomas Reid, Lawrence Y. Agodoa, Erin M. Bohen, Christina M. Yuan, Kevin C. Abbott, Thrombotic microangiopathy in United States long-term dialysis patients, Nephrology Dialysis Transplantation, Volume 21, Issue 1, January 2006, Pages 191–196, https://doi.org/10.1093/ndt/gfi153
Close - Share Icon Share
Abstract
Background. The incidence, risk factors, recurrence rates and prognosis of thrombotic microangiopathy (TMA) among long-term dialysis patients in the United States have not been previously described in a national population.
Methods. 272 024 Medicare primary patients in the United States Renal Data System (USRDS) initiated on end-stage renal disease (ESRD) therapy between 1 April 1995 and 31 December 1999 with Medicare as primary payer were analysed in a retrospective cohort study of USRDS of TMA. Cox regression was used to calculate adjusted hazard ratios (AHR) for risk of TMA and risk of death after TMA.
Results. The incidence of TMA in the first year of dialysis was 0.5% overall. Among patients with renal failure due to haemolytic uraemic syndrome (HUS), the incidence of TMA was highest in the first year of dialysis (HUS, 11.3% first year, 4.5% per year thereafter), while among patients without HUS the incidence of TMA was much lower and more constant over time (0.3% per year). In Cox regression analysis, independent risk factors for TMA were renal failure due to HUS (adjusted hazard ratio (AHR) 179, 95% CI 95–338), paediatric age (≤18 years vs older, AHR 2.59, 95% CI 1.48–4.55), female gender (AHR 1.99, 95% CI 1.43–2.78), and systemic lupus erythematosus (SLE, AHR 3.66, 95% CI 1.49–8.51). One-year survival after TMA was poor at 58% (AHR for mortality 2.04, 95% CI 1.23–3.38).
Conclusions. TMA is an uncommon cause of hospitalization after dialysis, but does recur in patients with HUS at a substantial rate. Younger age and SLE were risk factors for new onset TMA, which was associated with poor survival. Vigilant monitoring of select patients with HUS-related ESRD and higher-risk patients with SLE is warranted in the dialysis population.
Introduction
Thrombotic microangiopathy (TMA) is a microvascular occlusive disorder marked by predominantly platelet thrombi in the renal and/or systemic circulations. Haemolytic uraemic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP) are the clinical entities comprising TMA, with predominantly renal manifestations in the former, while the latter more often presents with systemic and neurologic findings. For most adult patients, diagnostic distinction is less important than timely treatment. Histopathologically, TTP and HUS are similar, and clinical overlap is common [1].
For both disorders, the incidence, risk factors, and prognosis for the non-renal population have been previously described. The reported incidence rate of HUS is variable by age, and ranges from 0.5–6.1 cases/100 000 persons annually [2]. Due to its rarity in adults, it is not tracked by the National Hospital Discharge Survey, and is routinely tracked only in children. It is the most common form of acute renal failure in children, and incidence falls with age. TTP is less common, with an annual incidence of 0.4 cases/100 000 persons [2]. The incidence of TMA in the renal transplant population has been reported to be 4.9 episodes per 1000 person-years at-risk [3]. With the exception of this last study, no analysis has reported incidence rates in person-years at-risk, making comparisons among analyses problematic. The primary risk factor for HUS is infection with shiga-toxin-producing strains of Escherichia coli, though a familial form has also been identified. Multiple risk factors for TTP in the non-dialysis population have been proposed (Table 1) [4–6]. Prognosis for TTP has improved dramatically since the routine use of plasma exchange and plasmapheresis therapies [7,8].
Reported risk factors for TMA (HUS and TTP) in the non-dialysis population
| Idiopathic |
| Familial |
| Infection (HIV, shiga-toxin-producing strains of E.coli, Rocky Mountain spotted fever) |
| Organ and bone marrow transplantation |
| Medications (calcineurin inhibitors, ticlodipine, clopidogrel, quinine, mitomycin, cisplatin, gemcitabine) |
| Malignancy |
| Pregnancy |
| Collagen vascular disease (SLE, rheumatoid arthritis, scleroderma) |
| Antiphospholipid antibody syndrome |
| Idiopathic |
| Familial |
| Infection (HIV, shiga-toxin-producing strains of E.coli, Rocky Mountain spotted fever) |
| Organ and bone marrow transplantation |
| Medications (calcineurin inhibitors, ticlodipine, clopidogrel, quinine, mitomycin, cisplatin, gemcitabine) |
| Malignancy |
| Pregnancy |
| Collagen vascular disease (SLE, rheumatoid arthritis, scleroderma) |
| Antiphospholipid antibody syndrome |
Reported risk factors for TMA (HUS and TTP) in the non-dialysis population
| Idiopathic |
| Familial |
| Infection (HIV, shiga-toxin-producing strains of E.coli, Rocky Mountain spotted fever) |
| Organ and bone marrow transplantation |
| Medications (calcineurin inhibitors, ticlodipine, clopidogrel, quinine, mitomycin, cisplatin, gemcitabine) |
| Malignancy |
| Pregnancy |
| Collagen vascular disease (SLE, rheumatoid arthritis, scleroderma) |
| Antiphospholipid antibody syndrome |
| Idiopathic |
| Familial |
| Infection (HIV, shiga-toxin-producing strains of E.coli, Rocky Mountain spotted fever) |
| Organ and bone marrow transplantation |
| Medications (calcineurin inhibitors, ticlodipine, clopidogrel, quinine, mitomycin, cisplatin, gemcitabine) |
| Malignancy |
| Pregnancy |
| Collagen vascular disease (SLE, rheumatoid arthritis, scleroderma) |
| Antiphospholipid antibody syndrome |
An Ovid Medline® literature search was conducted encompassing the period 1966 through the third week of July, 2005. Search terms included thrombotic microangiopathy, haemolytic uraemic syndrome, and thrombotic thrombocytopenic purpura combined sequentially with end-stage renal disease or dialysis. This search identified no studies enrolling primarily a dialysis cohort. While recurrent HUS has been described after kidney transplantation, there is currently no information available for counselling patients with end-stage renal disease attributed to HUS or TTP about risk of recurrence, risk factors associated with recurrence, or long-term prognosis. In the present study, we report the incidence and prognosis of TMA in the US chronic dialysis population, and identify potential risk factors in this cohort.
Subjects and methods
A national registry (the USRDS) was analysed in a historical cohort study of the rate, risk factors and mortality associated with TMA in end-stage renal disease (ESRD) patients. The variables included in the USRDS standard analysis files (SAFs), as well as data collection methods and validation studies, are listed at the USRDS website, under ‘Researcher's Guide to the USRDS Database’, Section E, ‘Contents of all the SAFs’ (Standard Analysis Files), and published in the USRDS (www.usrds.org). The demographics of the dialysis population have been previously described (2002 USRDS report). All hospitalizations with a primary or secondary discharge diagnosis of thrombotic microangiopathy were extracted from SAF.HOSP and merged with SAF.PATIENTS. The latter file was also used to extract the cause of renal disease (PDIS) and the cause and date of patient death. SAF.MEDEVID was used for additional information coded in the medical evidence form starting in 1995, and has been validated for use in research [9]. Therefore, patients who presented to ESRD on or after 1 April 1995 until 31 December 1999 were selected for the study and followed through 31 December 2000 for a diagnosis of TMA, and until 30 September 2001 for death.
Outcomes included hospitalizations with a primary or secondary discharge diagnosis of thrombotic microangiopathy (TMA, International Classification of Diseases, 9th Revision (ICD9) codes 283.11x or 446.6x, coded 1 if present, 0 if absent). We chose to limit the extraction to the primary or secondary discharge diagnosis because of potential inaccuracies in regional reporting of TMA in the Physicians supplier and Institutional Claims files (personal communication to Dr Paul Eggers). Only ICD9 procedure codes, and not Current Procedural Terminology (CPT) codes, were available from the database and were not considered reliable for reporting performance of therapeutic plasma exchange. Cases of TMA occurring after renal transplantation were not considered an outcome for purposes of this study (i.e. coded as 0). Other outcomes included death from any cause. Time to diagnosis of TMA was defined as the time from the first dialysis session until the date of discharge diagnosis for TMA, with patients censored for death, transplantation, or the end of the study period (31 December 2000), whichever came first. Time to death was defined as time after the date of diagnosis of TMA until death, censored for the end of the study period, in this case considered 30 September 2001.
All analyses were performed using SPSS 12.0™ (SPSS, Inc., Chicago, IL). Files were merged and converted to SPSS files using DBMS/Copy (Conceptual Software, Houston, TX). Statistical significance was defined as P<0.05. Univariate analysis was performed with chi-square testing for categorical variables (Fisher exact test for violations of Cochran's assumptions) and Student's t-test (Mann–Whitney for non-normal distributions) for continuous variables. Variables with P<0.10 in univariate analysis for a relationship with TMA (including age, gender, year of first dialysis session, and history of transplant, diabetes, or HUS as cause of ESRD) were entered into multivariate analysis as covariates. An exception was made for factors with a potential biological relationship with TMA [10]. These additional variables include race, BMI, smoking status, use of Epo at the time of dialysis initiation, haematocrit at the time of dialysis initiation, and history of alcoholism or SLE.
Cox regression models were performed after testing associations with TMA, using covariates as specified above. Patient survival curves were calculated using life table analysis with patients censored at the time of loss to follow-up. Time to TMA was calculated as the time from the date of dialysis initiation until the diagnosis date of TMA, censored for death, end of the study period, loss to follow-up, or renal transplantation. Survival was calculated from the date of TMA diagnosis until death, censored for last follow-up visit, or loss to follow-up occurring after diagnosis. Covariates were as for univariate analysis. The association of TMA with survival was calculated as a time-dependent variable in Cox regression, with all values prior to TMA coded as 0 (including patients who were never diagnosed with TMA, all values after diagnosis as 1).
Results
From 1 April 1995 to 31 December 1999, 366 738 patients started long-term dialysis. Of these, 272 024 were documented as having Medicare as primary payer at the time of the first dialysis session. This cohort therefore comprised the study population.
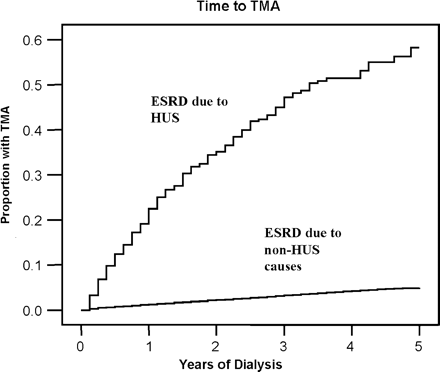
There were 215 cases of TMA during the study period; among those coded as a primary diagnosis, 20 were coded as HUS and 35 coded as TMA unspecified; among those coded as secondary diagnoses, 107 were coded as HUS and 54 coded as TMA unspecified (one patient was coded as TMA primary and HUS secondary). The overall incidence in the cohort was 0.5% annually. The incidence of TMA was the highest in the first year of dialysis among patients with renal failure due to HUS, occurring in 49 patients (11.3%) at one year and in approximately 20 patients (4.5%) annually thereafter, while the incidence of TMA was more constant over time among patients without HUS (815 patients per year, or 0.3% annually), as shown in Figure 1.
Life table plot of time to thrombotic microangiopathy (TMA), stratified by end-stage renal disease (ESRD) due to haemolytic uraemic syndrome (HUS) vs all other causes of renal failure. Patients with HUS as a cause of renal failure had a high rate of recurrence in the first year, at 11.3%, and 4.5% per year afterward. In contrast, patients without HUS had a much lower rate of TMA (de novo TMA, 0.3% per year) and the rate was relatively constant over time.
Factors assessed in the study population and results of univariate analysis are shown in Table 2. Variables with statistically significant associations with TMA incidence include female gender, paediatric age, HUS, SLE, glomerulonephritis as cause of ESRD and a history of illicit drug use. Diabetes as cause of ESRD was associated with a reduced incidence of TMA in univariate analysis. In addition to the factors listed in Table 2, there were no statistically significant differences in incidence of TMA by state, region of country, or quartiles of albumin (data not shown).
Factors assessed in US long-term dialysis patients, 1 April 1995 to 31 December 1999
| Factor . | Dialysis patients hospitalized for TMA . | All other dialysis patients . | ||
|---|---|---|---|---|
| 215 (0.1%) | 271 809 (99.9%) | |||
| N with valid causes of ESRD | ||||
| Female | 141 (65.6%)a | 128 330 (47.2%) | ||
| African American | 72 (33.5%) | 81 238 (29.9%) | ||
| (vs other races) | ||||
| Mean age (years) | 46.1±20.7 | 62.7±15.9 | ||
| Age ≤18 years | 18 (8.4%) | 1958 (0.7%) | ||
| Body mass index (kg/m2) | 24.9±5.6 | 25.8±5.9b | ||
| Cause of ESRDc | ||||
| HUS (ICD 283.11)d | 73 (30.7%)a | 361 (0.001%) | ||
| Glomerulonephritis | 101 (47.6%)a | 28 304 (10.7%) | ||
| Diabetes | 33 (15.6%)a | 116 678 (44.3%) | ||
| Systemic lupus erythematosus | 10 (4.7)a | 2837 (1.1) | ||
| Categorical variables from the medical evidence form (2728), history of | ||||
| Smoking | 14 (6.6%) | 14 837 (5.6%) | ||
| Alcohol use | 5 (2.4%) | 4064 (1.5%) | ||
| Illicit drug use | 7 (3.3%)a | 2300 (0.9%) | ||
| HIV+e | 1 (1.9%) | 1718 (2.6%) | ||
| Cancer | 7 (3.3%) | 13 704 (5.0%) | ||
| Dialysis modalityc | ||||
| Haemodialysis | 191 (90.1%) | 237 224 (90.1%) | ||
| Peritoneal dialysis | 21 (9.9%) | 26 000 (9.9%) | ||
| Haematocrit (%) | 25.2±8.7 | 26.8±8.5b | ||
| Serum creatinine (mg/dl) | 8.8±4.2 | 8.1±3.8b | ||
| Serum albumin (g/l) | 3.2±0.7 | 3.2±0.7 | ||
| Factor . | Dialysis patients hospitalized for TMA . | All other dialysis patients . | ||
|---|---|---|---|---|
| 215 (0.1%) | 271 809 (99.9%) | |||
| N with valid causes of ESRD | ||||
| Female | 141 (65.6%)a | 128 330 (47.2%) | ||
| African American | 72 (33.5%) | 81 238 (29.9%) | ||
| (vs other races) | ||||
| Mean age (years) | 46.1±20.7 | 62.7±15.9 | ||
| Age ≤18 years | 18 (8.4%) | 1958 (0.7%) | ||
| Body mass index (kg/m2) | 24.9±5.6 | 25.8±5.9b | ||
| Cause of ESRDc | ||||
| HUS (ICD 283.11)d | 73 (30.7%)a | 361 (0.001%) | ||
| Glomerulonephritis | 101 (47.6%)a | 28 304 (10.7%) | ||
| Diabetes | 33 (15.6%)a | 116 678 (44.3%) | ||
| Systemic lupus erythematosus | 10 (4.7)a | 2837 (1.1) | ||
| Categorical variables from the medical evidence form (2728), history of | ||||
| Smoking | 14 (6.6%) | 14 837 (5.6%) | ||
| Alcohol use | 5 (2.4%) | 4064 (1.5%) | ||
| Illicit drug use | 7 (3.3%)a | 2300 (0.9%) | ||
| HIV+e | 1 (1.9%) | 1718 (2.6%) | ||
| Cancer | 7 (3.3%) | 13 704 (5.0%) | ||
| Dialysis modalityc | ||||
| Haemodialysis | 191 (90.1%) | 237 224 (90.1%) | ||
| Peritoneal dialysis | 21 (9.9%) | 26 000 (9.9%) | ||
| Haematocrit (%) | 25.2±8.7 | 26.8±8.5b | ||
| Serum creatinine (mg/dl) | 8.8±4.2 | 8.1±3.8b | ||
| Serum albumin (g/l) | 3.2±0.7 | 3.2±0.7 | ||
Data given as the number (% of total) or mean ± one SD.
SLE, systemic lupus erythematosus; HUS, haemolytic uraemic syndrome; ESRD, end-stage renal disease; TMA, thrombotic microangiopathy.
To convert creatinine from mg/dl to µmol/l multiply by 88.
aP<0.05 by chi-square testing.
bP<0.05 by students T-test.
cInformation missing for 3% of study population.
dOnly HUS, not TMA, is listed as a cause of ESRD.
eInformation missing for 75.4% of patients.
Factors assessed in US long-term dialysis patients, 1 April 1995 to 31 December 1999
| Factor . | Dialysis patients hospitalized for TMA . | All other dialysis patients . | ||
|---|---|---|---|---|
| 215 (0.1%) | 271 809 (99.9%) | |||
| N with valid causes of ESRD | ||||
| Female | 141 (65.6%)a | 128 330 (47.2%) | ||
| African American | 72 (33.5%) | 81 238 (29.9%) | ||
| (vs other races) | ||||
| Mean age (years) | 46.1±20.7 | 62.7±15.9 | ||
| Age ≤18 years | 18 (8.4%) | 1958 (0.7%) | ||
| Body mass index (kg/m2) | 24.9±5.6 | 25.8±5.9b | ||
| Cause of ESRDc | ||||
| HUS (ICD 283.11)d | 73 (30.7%)a | 361 (0.001%) | ||
| Glomerulonephritis | 101 (47.6%)a | 28 304 (10.7%) | ||
| Diabetes | 33 (15.6%)a | 116 678 (44.3%) | ||
| Systemic lupus erythematosus | 10 (4.7)a | 2837 (1.1) | ||
| Categorical variables from the medical evidence form (2728), history of | ||||
| Smoking | 14 (6.6%) | 14 837 (5.6%) | ||
| Alcohol use | 5 (2.4%) | 4064 (1.5%) | ||
| Illicit drug use | 7 (3.3%)a | 2300 (0.9%) | ||
| HIV+e | 1 (1.9%) | 1718 (2.6%) | ||
| Cancer | 7 (3.3%) | 13 704 (5.0%) | ||
| Dialysis modalityc | ||||
| Haemodialysis | 191 (90.1%) | 237 224 (90.1%) | ||
| Peritoneal dialysis | 21 (9.9%) | 26 000 (9.9%) | ||
| Haematocrit (%) | 25.2±8.7 | 26.8±8.5b | ||
| Serum creatinine (mg/dl) | 8.8±4.2 | 8.1±3.8b | ||
| Serum albumin (g/l) | 3.2±0.7 | 3.2±0.7 | ||
| Factor . | Dialysis patients hospitalized for TMA . | All other dialysis patients . | ||
|---|---|---|---|---|
| 215 (0.1%) | 271 809 (99.9%) | |||
| N with valid causes of ESRD | ||||
| Female | 141 (65.6%)a | 128 330 (47.2%) | ||
| African American | 72 (33.5%) | 81 238 (29.9%) | ||
| (vs other races) | ||||
| Mean age (years) | 46.1±20.7 | 62.7±15.9 | ||
| Age ≤18 years | 18 (8.4%) | 1958 (0.7%) | ||
| Body mass index (kg/m2) | 24.9±5.6 | 25.8±5.9b | ||
| Cause of ESRDc | ||||
| HUS (ICD 283.11)d | 73 (30.7%)a | 361 (0.001%) | ||
| Glomerulonephritis | 101 (47.6%)a | 28 304 (10.7%) | ||
| Diabetes | 33 (15.6%)a | 116 678 (44.3%) | ||
| Systemic lupus erythematosus | 10 (4.7)a | 2837 (1.1) | ||
| Categorical variables from the medical evidence form (2728), history of | ||||
| Smoking | 14 (6.6%) | 14 837 (5.6%) | ||
| Alcohol use | 5 (2.4%) | 4064 (1.5%) | ||
| Illicit drug use | 7 (3.3%)a | 2300 (0.9%) | ||
| HIV+e | 1 (1.9%) | 1718 (2.6%) | ||
| Cancer | 7 (3.3%) | 13 704 (5.0%) | ||
| Dialysis modalityc | ||||
| Haemodialysis | 191 (90.1%) | 237 224 (90.1%) | ||
| Peritoneal dialysis | 21 (9.9%) | 26 000 (9.9%) | ||
| Haematocrit (%) | 25.2±8.7 | 26.8±8.5b | ||
| Serum creatinine (mg/dl) | 8.8±4.2 | 8.1±3.8b | ||
| Serum albumin (g/l) | 3.2±0.7 | 3.2±0.7 | ||
Data given as the number (% of total) or mean ± one SD.
SLE, systemic lupus erythematosus; HUS, haemolytic uraemic syndrome; ESRD, end-stage renal disease; TMA, thrombotic microangiopathy.
To convert creatinine from mg/dl to µmol/l multiply by 88.
aP<0.05 by chi-square testing.
bP<0.05 by students T-test.
cInformation missing for 3% of study population.
dOnly HUS, not TMA, is listed as a cause of ESRD.
eInformation missing for 75.4% of patients.
In Cox regression analysis, only four factors were independently associated with TMA: HUS and systemic lupus as causes of ESRD, younger (and especially paediatric age), and female gender. Table 3 shows the results of Cox regression analysis.
Cox regression analysis of factors associated with hospitalizations for TMA after initiation of dialysis, censored for renal transplantationa
| Factor . | Adjusted hazard ratio for TMA (95% confidence interval) . | |
|---|---|---|
| HUS as cause of ESRD | 179 (95–338)b | |
| Female gender (vs male) | 1.99 (1.43–2.77)b | |
| Age | ||
| Age ≤18 (vs older) | 2.59 (1.48–4.55)b | |
| Quartile 1 (≤52.6 years) | 3.27 (1.87–5.71)b | |
| Quartile 2 (>52.6–65.8 years) | 2.37 (1.32–4.25)b | |
| Quartile 3 (>65.8–74.7 years) | 1.67 (0.90–3.12) | |
| Quartile 4 (>74.7 years) | 1.00 (reference) | |
| Data from CMS form 2728 (at time of dialysis initiation) | ||
| SLE (history of) | 3.66 (1.49–8.51)b | |
| Factor . | Adjusted hazard ratio for TMA (95% confidence interval) . | |
|---|---|---|
| HUS as cause of ESRD | 179 (95–338)b | |
| Female gender (vs male) | 1.99 (1.43–2.77)b | |
| Age | ||
| Age ≤18 (vs older) | 2.59 (1.48–4.55)b | |
| Quartile 1 (≤52.6 years) | 3.27 (1.87–5.71)b | |
| Quartile 2 (>52.6–65.8 years) | 2.37 (1.32–4.25)b | |
| Quartile 3 (>65.8–74.7 years) | 1.67 (0.90–3.12) | |
| Quartile 4 (>74.7 years) | 1.00 (reference) | |
| Data from CMS form 2728 (at time of dialysis initiation) | ||
| SLE (history of) | 3.66 (1.49–8.51)b | |
SLE, systemic lupus erythematosus; HUS, haemolytic uraemic syndrome; ESRD, end-stage renal disease; TMA, thrombotic microangiopathy.
aVariables with P<0.10 in univariate analysis for a relationship with TMA (age, gender, year of first dialysis session and history of transplant, diabetes or HUS as cause of ESRD) were entered into multivariate analysis as covariates. An exception was made for factors (race, BMI, smoking status, use of Epo at time of dialysis initiation, haematocrit at time of dialysis initiation and history of alcoholism or SLE) with a potential biological relationship with TMA.
bP<0.05.
Cox regression analysis of factors associated with hospitalizations for TMA after initiation of dialysis, censored for renal transplantationa
| Factor . | Adjusted hazard ratio for TMA (95% confidence interval) . | |
|---|---|---|
| HUS as cause of ESRD | 179 (95–338)b | |
| Female gender (vs male) | 1.99 (1.43–2.77)b | |
| Age | ||
| Age ≤18 (vs older) | 2.59 (1.48–4.55)b | |
| Quartile 1 (≤52.6 years) | 3.27 (1.87–5.71)b | |
| Quartile 2 (>52.6–65.8 years) | 2.37 (1.32–4.25)b | |
| Quartile 3 (>65.8–74.7 years) | 1.67 (0.90–3.12) | |
| Quartile 4 (>74.7 years) | 1.00 (reference) | |
| Data from CMS form 2728 (at time of dialysis initiation) | ||
| SLE (history of) | 3.66 (1.49–8.51)b | |
| Factor . | Adjusted hazard ratio for TMA (95% confidence interval) . | |
|---|---|---|
| HUS as cause of ESRD | 179 (95–338)b | |
| Female gender (vs male) | 1.99 (1.43–2.77)b | |
| Age | ||
| Age ≤18 (vs older) | 2.59 (1.48–4.55)b | |
| Quartile 1 (≤52.6 years) | 3.27 (1.87–5.71)b | |
| Quartile 2 (>52.6–65.8 years) | 2.37 (1.32–4.25)b | |
| Quartile 3 (>65.8–74.7 years) | 1.67 (0.90–3.12) | |
| Quartile 4 (>74.7 years) | 1.00 (reference) | |
| Data from CMS form 2728 (at time of dialysis initiation) | ||
| SLE (history of) | 3.66 (1.49–8.51)b | |
SLE, systemic lupus erythematosus; HUS, haemolytic uraemic syndrome; ESRD, end-stage renal disease; TMA, thrombotic microangiopathy.
aVariables with P<0.10 in univariate analysis for a relationship with TMA (age, gender, year of first dialysis session and history of transplant, diabetes or HUS as cause of ESRD) were entered into multivariate analysis as covariates. An exception was made for factors (race, BMI, smoking status, use of Epo at time of dialysis initiation, haematocrit at time of dialysis initiation and history of alcoholism or SLE) with a potential biological relationship with TMA.
bP<0.05.
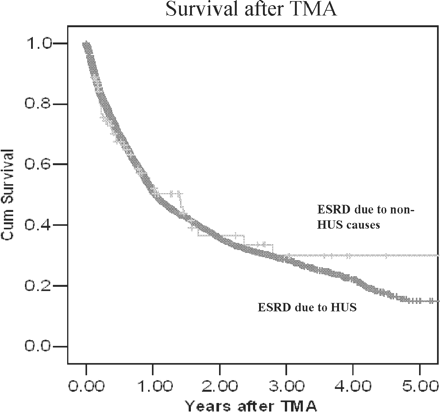
Figure 2 shows survival after TMA for patients with ESRD due to HUS and for those with ESRD due to all other causes. Regardless of the cause of ESRD, the survival was 58% at one year after the diagnosis of TMA. In time-dependent Cox regression, TMA was independently associated with an increased risk of death (adjusted hazard ratio, 2.04; 95% CI, 1.24–3.39). The leading causes of death after TMA were cardiovascular disease (29%), followed by infectious death (14.3%) and cancer-related death (3.2%). These percentages were not substantially different from causes of death among patients who were not diagnosed with TMA.
Survival after TMA did not differ between those with HUS and other causes of renal failure.
Discussion
We describe the incidence, risk factors, recurrence rates and survival of patients with TMA in the United States long-term dialysis population. The incidence of TMA after the onset of dialysis has not been previously described. The rate of recurrence of TMA in dialysis patients with HUS as the cause of ESRD was quite high, particularly in the first year after initiation of dialysis. This was not the case with de novo TMA, in which the rate was much lower and stable over time. Identified risk factors for TMA in this population were similar to those reported for the non-dialysis population, though some previously identified factors, such as malignancy, were not observed in our study. Not surprisingly, given the burden of co-morbid illness in the dialysis population, mortality after TMA was higher than that reported in the non-dialysis population.
Incidence of TMA
Shumak et al. and Hayward et al. have reported relapse rates up to 30% of patients with TMA, some of which occurred years after successful treatment of the initial episode [11,12]. It is possible that differential rates of plasmapheresis and exchange therapies between this cohort and historical comparison groups are responsible for the improved long-term survival of dialysis patients with TMA. Unfortunately, the USRDS database does not reliably track the performance of therapeutic plasma exchange, and it could not be determined whether plasma exchange was associated with improved survival in such patients. In any case, the indications and contraindications for plasma exchange were not available (for example, plasma exchange might not be performed in patients with mild disease), and therefore interpretation of outcomes of patients in whom plasma exchange was used would be problematic.
Only one study of TMA in ESRD (dialysis or transplant) has reported rates of TMA in person-years at-risk, allowing for comparison [4]. While this analysis was limited to TMA in the post-renal transplant population, the comparison may provide intriguing insights into the pathophysiology of TMA. While we report a TMA recurrence rate of 11.3% in the first year in the dialysis population, vs 0.3% of de novo TMA in those with ESRD due to causes other than HUS, Reynolds reported a recurrence rate (post-transplant) of 15% at 1 year in patients with ESRD due to HUS, vs a de novo rate of 0.4% in patients with ESRD due to non-HUS causes [4]. While it is tempting to attribute the higher rates in the transplant population to prevalent use of immunosuppressive medications, some of which have been implicated as potential causes of TMA, several points argue against this. First, Reynolds et al. used all discharge diagnoses rather than primary or secondary diagnoses, as in our study. It is, therefore, possible that our data under-reports the true incidence of TMA in the dialysis population. Second, the rate of de novo TMA was similar in the transplant population to the rate in our cohort; if immunosuppressive regimens (primarily those including calcineurin inhibitors) were causative, we might expect de novo TMA rates to be higher in the transplant group. In fact, given the widely held belief that calcineurin inhibitors are associated with TMA, the similarity of the de novo rates of TMA between the dialysis and transplant populations is rather striking.
Risk factors for TMA
We identified similar potential risk factors for TMA as in the non-dialysis population. Younger age, female gender and a history of systemic lupus erythematosus were all independent predictors of TMA in our study. The increased risk of developing TMA in patients with SLE has been recognized for some time, though the pathophysiologic mechanism underlying the relationship has not been well defined. The higher prevalence of anti-phospholipid antibodies (which in turn have been shown to cause a microangiopathic syndrome) in patients with SLE may be in part responsible for this association [13]. In a case report and literature review Musio et al. have hypothesized that SLE and TMA patients share several additional features, such as common auto-immune mechanisms, platelet abnormalities, and fibrinolytic disorders, all of which (alone or in combination) may account for the association [14].
Malignancy was not identified as a potential risk factor in our analysis, though other studies have identified multiple cancers and chemotherapeutic agents (which we did not examine) as risk factors for TMA [5,15]. The reasons for this finding are unclear and impossible to deduce from our retrospective analysis, though may be related to differences in rates of higher-TMA risk cancer types, in rates of chemotherapy use between the two populations, or other population differences we did not examine. We did not find a significant association between HIV seropositivity and TMA, as has been reported in the pre-HAART era [16]. However, in the HAART era, TMA in association with HIV has become rare [17]. Finally, an important negative finding in our study was the lack of difference in rates of TMA between those on haemodialysis compared with those on peritoneal dialysis. This may be useful information for counselling patients with ESRD due to TMA about their options for renal replacement therapy.
Survival of ESRD patients with TMA
Survival after TMA was poor in our analysis, particularly in comparison to reported studies in the non-dialysis population, where mortality has improved dramatically (reported as less than 10%) since plasmapheresis and exchange therapies have become standards of care [9]. This difference may be due to several factors. One possibility may be under-recognition of TMA in the dialysis population, where anaemia is prevalent and co-morbid conditions may cloud identification of sub-clinical illness. Another possibility is that the burden of co-morbid disease in the dialysis population negatively impacts survival when compared with a non-dialysis population that is typically healthier.
There are a number of important limitations of our study. As with all retrospective analyses, our findings are associative; it would be misleading to assign causality to our identified potential risk factors. In addition, the diagnosis of TMA in our analysis is limited to hospital discharge diagnoses, either primary or secondary; those patients with sub-clinical disease or those patients dying prior to hospitalization from complications of TMA are not included in our analysis and may lead to under-reporting in our study. With respect to the diagnosis of TMA, our study design does not allow us to verify clinic details of the diagnosis, which may introduce unpredictable errors into our findings. Likewise, limitations of the registry preclude making prognostic or other distinctions between subtypes of TMA. For example, shiga-toxin-associated HUS and non-shiga-toxin HUS have different prognoses as well as different associated risk factors; coding limitations preclude clarification of these distinctions. It is also important to note that our study cannot distinguish between relapse and recurrence, both of which occur frequently in the non-dialysis population.
Our findings emphasize the need for ongoing close surveillance of patients with ESRD due to HUS. Serial monitoring of the haematocrit, platelet count and lactate dehydrogenase levels, in addition to surveillance for recurrence of symptoms, has been recommended in the non-dialysis population. While there is no consensus regarding a standard surveillance schedule in the dialysis population, our findings suggest that more frequent clinical monitoring of patients with HUS as the cause of ESRD should be undertaken, particularly in the first year after initiation of dialysis. Certain populations are known to be at higher risk of recurrence than others, and careful surveillance of these patients would be prudent [18].
In conclusion, we report a substantial rate of recurrent TMA in the dialysis population, in which survival is noted to be poor and risk factors similar to the non-dialysis population. However, de novo TMA is uncommon. Similar to the non-ESRD population, TMA occurred primarily in younger patients, with females and patients with systemic lupus erythematosus at particular risk. Whether better recognition of TMA in the dialysis population would yield improvements in survival is unknown. Given the paucity of information in this patient population, and the relatively limited disease incidence, creation of a dedicated clinical registry for research purposes is indicated.
Conflict of interest statement. The authors report no conflict of interest related to any aspect of study concept, design, analysis or reporting. The opinions are solely those of the authors and do not represent an endorsement by the Department of Defense or the National Institutes of Health. This is a US Government work. There are no restrictions on its use.
References
Ruggenenti P, Remuzzi G. Pathophysiology and management of thrombotic microangiopathies.
Reynolds J, Agodoa L, Yuan C, Abbott K. Thrombotic microangiopathy after renal transplantation in the United States.
Lesesne JB, Rothschild N, Erickson B. Cancer-associated hemolytic uremic syndrome: Analysis of 85 cases from a national registry.
Nesher G, Vaughn E, Terry L et al. Thrombotic microangiopathic hemolytic anemia in systemic lupus erythematosus.
Weiner C. Thrombotic microangiopathy in pregnancy and the postpartum period.
Rock G, Shumak K, Buskard N et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.
Von Baeyer. Plasmapheresis in thrombotic microangiopathy-associated syndromes: review of outcome data derived from clinical trials and open studies.
Longenecker JC, Coresh J, Klag MJ et al. Validation of co-morbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD.
Szklo M, Nieto FJ.
Shumak K, Rock G, Nair R. Late relapses in patients successfully treated for thromobotic thrombocytopenic purpura.
Hayward C, Sutton D, Carter WH et al. Treatment outcomes in patients with adult thrombotic thrombocytopenic purpura—hemolytic uremic syndrome.
Nzerue CM, Hewan-Lowe K, Pierangeli S, Harris EN. “Black swan in the Kidney”: Renal involvement in the antiphospholipid antibody syndrome.
Musio F, Bohen EM, Yuan CM, Welch PG. Review of thrombotic thrombocytopenic purpura in the setting of systemic lupus erythematosus.
Jackson AM, Rose BD, Graff LG et al. Thrombotic microangiopathy and renal failure associated with antineoplastic chemotherapy.
Ahmed S, Siddiqui RK, Siddiqui AK, Zaidi SA, Cervia J. HIV associated thrombotic microangiopathy.
Becker S, Fusco G, Fusco J et al. Collaborations in HIV Outcomes Research/US Cohort. HIV-associated thrombotic microangiopathy in the era of highly active antiretroviral therapy: an observational study.
Author notes
1Nephrology Service and 4Hematology/Oncology Service, Walter Reed Army Medical Center, Washington and Uniformed Services University of the Health Sciences, Bethesda, MD, 2Nephrology Service, Brooke Army Medical Center, Fort Sam Houston, TX, 3Nephrology Service, University of Texas, Galveston and 5NIDDK, NIH, Bethesda, MD, USA





Comments