-
PDF
- Split View
-
Views
-
Cite
Cite
Knut Aasarød, Bjarne M. Iversen, Jens Hammerstrøm, Leif Bostad, Lars Vatten, Størker Jørstad, Wegener's granulomatosis: clinical course in 108 patients with renal involvement, Nephrology Dialysis Transplantation, Volume 15, Issue 5, May 2000, Pages 611–618, https://doi.org/10.1093/ndt/15.5.611
Close - Share Icon Share
Abstract
Background. The aim of this study was to evaluate the clinical course of patients with Wegener's granulomatosis and renal involvement, with special reference to relapse rate, renal and patient survival and morbidity from serious infections.
Methods. A retrospective analysis was carried out of 108 patients presenting with Wegener's granulomatosis and active renal disease in eight hospitals in Norway between 1988 and 1998. Multivariate analysis was used to investigate whether selected variables predicted relapse, renal and patient survival and serious infections.
Results. Median follow‐up was 41.5 months. Twenty‐two patients (20.4%) were admitted with a need for dialysis. Complete remission was obtained in 81.5% after a median of 4 months, and 54.7% relapsed after a median of 22.5 months. Two‐ and five‐year renal survival was 86 and 75%, respectively, and 22.8% developed end‐stage renal disease (ESRD). Two‐ and five‐year patient survival was 88 and 74%, respectively, and the cumulative mortality was 3.8 times higher than expected. The relative risk of relapse increased with the use of intravenous pulse cyclophosphamide compared with daily oral cyclophosphamide. Initial renal function predicted renal survival, and low serum albumin and high age at treatment start increased the mortality risk. Thirty one per cent of the patients were hospitalized for serious infections during follow‐up. Old age increased the risk of having an infection.
Conclusions. The current treatment of Wegener's granulomatosis does not prevent relapse, development of ESRD and serious treatment‐induced infections in a considerable fraction of the patients. Alternative strategies for the management of this disease will be an important objective for further studies.
Introduction
Wegener's granulomatosis is a necrotizing granulomatous small‐vessel vasculitis that affects mainly the upper and lower respiratory tract and the kidneys. Although the prognosis has improved dramatically over the last decades, the disease still carries a high mortality and morbidity rate. In the 1950s, the median patient survival was 5 months, and 1 year mortality was 80% [1]. The introduction of aggressive treatment with high doses of glucocorticosteroids combined with cyclophosphamide has improved the prospects for these patients. Fauci and co‐workers induced remission in 93% out of 85 patients with Wegener's granulomatosis, and 88% were still alive after a mean follow‐up of 51 months [2]. In the classical study by Hoffman and co‐workers, 80% of the 158 patients were alive after 8 years [3].
The epidemiology of Wegener's granulomatosis is largely unknown. The incidence is low, diagnostic criteria vary and studies often come from tertiary referral hospitals introducing the possibility of selection bias. In the 1970s, the reported annual incidence was between 0.4 and 4 per million [4,5]. The estimates have increased over the last decade, which may be attributed to an increased awareness of the disease among clinicians, but also to the introduction of assays for anti‐neutrophil cytoplasmic autoantibodies (ANCA) in 1985 [6]. Thus, a British study from 1995 reported an annual incidence of 8.5 per million [7].
The proportion of patients with renal involvement at disease presentation has varied between studies from <20% to 80%, but invariably increases to 80–94% during follow‐up [3,8]. Renal involvement in Wegener's granulomatosis heralds a more severe outcome. In a study comparing the clinical course of ‘renal’ and ‘non‐renal’ Wegener's granulomatosis, the only deaths occurred in the group with renal involvement [9]. In another study, 1 year survival was 62% in 43 patients with microscopic polyangiitis and Wegener's granulomatosis with renal involvement [10]. This is in contrast to more recent reports documenting a very good survival in spite of renal disease [11,12].
The glomerulonephritis characteristic of Wegener's granulomatosis is crescentic, necrotizing and with little or no immune deposits. These changes are not specific for Wegener's granulomatosis, and can also be seen in other vasculitides such as microscopic polyangiitis and Churg Strauss disease. They also occur in idiopathic necrotizing crescentic glomerulonephritis (NCGN) without evidence of extrarenal vasculitis.
In the present study, we report the clinical course of 108 patients with Wegener's granulomatosis and renal involvement, with special emphasis on relapse rate, the development of end‐stage renal disease (ESRD), patient survival and occurrence of serious infections.
Subjects and methods
Patients
One hundred and eight patients with Wegener's granulomatosis and active renal disease who were treated in eight hospitals in Norway between 1988 and 1998 were included in this study. Each of the eight hospitals treated all patients with Wegener's granulomatosis in its catchment area. The total catchment area for all the hospitals comprised ∼2.2 million inhabitants, or ∼50% of the total population of Norway. All hospitals had an electronically based register for patient diagnosis, and records from these registers were used to find patients with the diagnosis of Wegener's granulomatosis. The information was then scrutinized by one of the authors (K.A.) to confirm the diagnosis. The review covered all medical information available for each patient, and included an interview with the physicians who treated the patients. Autopsy reports were also obtained when they were available.
Clinical diagnosis
The diagnosis of Wegener's granulomatosis was based on the clinical criteria developed by the American College of Rheumatology, with at least two of the following criteria fulfilled: oral ulcers or nasal discharge, abnormal findings on chest radiograph (nodules, cavities or fixed infiltrates), abnormal urinary sediment (red cell casts or >5 red blood cells per high‐power field) or granulomatous inflammation on biopsy [13]. As an additional requirement, patients without a granulomatous inflammation shown on biopsy had to be seropositive for ANCA. A clinical diagnosis of active renal disease was defined as signs of active urine sediment, i.e. >5 erythrocytes per high‐power field (magnification, ×400), or erythrocyte or granular casts.
Renal biopsies
For 95 of the 108 patients, a percutaneous renal biopsy was performed and evaluated by two pathologists; one from the referral hospital and by one of the authors (L.B.), a nephropathologist.
Clinical and laboratory investigation
Inclusion time (initial evaluation) was defined as the time of kidney biopsy. For the 13 patients who did not have a kidney biopsy, inclusion was recorded as the time of their first hospital admission for an active Wegener's granulomatosis with renal involvement. Clinical and laboratory data at inclusion, after 1 year and at the last visit before March 1999 were collected. For those who died, the clinical and laboratory status at the last visit before death was recorded. Routine laboratory tests were analysed at the department of clinical chemistry at each hospital. ANCAs were defined as either cytoplasmic (c‐ANCA) or perinuclear (p‐ANCA) and determined by indirect immunofluorescence microscopy [6]. The first organs affected at disease onset were recorded, as were all organs involved at inclusion time. The time span from the onset of the first symptoms related to Wegener's granulomatosis and inclusion was also recorded.
Disease activity
Disease activity was defined as the presence of any of the following: (i) typical histological abnormalities seen on biopsy of a clinically involved organ; (ii) progression of upper or lower airway or ocular disease in the absence of infection or other illness; (iii) progressive renal functional impairment as determined by active urinary sediment including red blood cell casts; (iv) progressive polyneuropathy, non‐vasculitic causes having been ruled out; and (v) if a patient had none of the above but had an elevated erythrocyte sedimentation rate, constitutional symptoms, fever or arthralgias/myalgias not related to identifiable non‐vasculitic processes, the patient was also considered to have an active disease [14].
Complete remission was defined as a state with no sign of active vasculitic disease and complete resolution of pulmonary infiltrates, improvement of renal function and resolution of extrarenal manifestation of vasculitis. The term partial remission was defined as a clear‐cut suppression of the progression of disease activity with stabilization of renal abnormalities, both functional and urinary findings, and partial resolution of pulmonary infiltrates. There should be no further worsening of other organ system disease activity, and there should be a progression towards improvement. Disease relapse was defined as the occurrence of any of the items i–v after having reached complete or partial remission.
Study end points
End points included death and ESRD, defined as a loss of renal function necessitating chronic dialysis or transplantation. Patients not reaching an end point were followed until their most recent hospital visit before March 1999. The time from inclusion to the first remission and from inclusion to the first relapse was recorded, as were the occurrence of infections leading to hospital treatment and the development of malignant diseases. The cause of death was considered to be Wegener's granulomatosis if the patient died from active disease or from side effects of the treatment.
Statistical analysis
Relapse‐free survival, renal (ESRD‐free) survival and patient survival were described with the Kaplan–Meier method. The Mann–Whitney U‐test was used to compare continuous variables, and the χ2 test was used to determine categorical variables between groups. The mortality risk ratio (MRR) of the 108 patients was calculated in a Cox proportional hazard model using all residents of Nord‐Trøndelag county, >20 years in 1984 as controls (n=87 285). The analysis was controlled for age and gender. An MRR of 1 indicates that the observed number of deaths equals the expected number. The mortality analysis was performed in collaboration with the Nord‐Trøndelag Health Study. Cox's proportional hazards regression analysis was used to investigate whether selected variables predicted renal and patient survival and relapse. Only patients surviving the acute phase of the disease (3 months after inclusion) were considered to be at risk of developing relapse or ESRD. Variables tested for relapse were age, gender, the number of organs affected, serum creatinine at entry and the route of cyclophosphamide administration (i.v./p.o.). For renal survival, variables tested were age, gender, time from disease presentation to inclusion, serum creatinine at the start of the study, blood thrombocyte count, proteinuria (g/24 h), the occurrence of relapse, the cumulative dose of cyclophosphamide given in the first year, the route of cyclophosphamide administration, plasma exchange and dialysis at study start. For patient survival, age, gender, creatinine at study start, serum albumin, blood haemoglobin, blood C‐reactive protein, erythrocyte sedimentation rate, infections requiring hospital treatment, the cumulative dose of cyclophosphamide and dialysis at study start were the variables tested. For infections requiring hospital treatment, age, serum creatinine at entry and the cumulative dose of cyclophosphamide were the variables used. The level of significance used was P<0.05, and all tests were two‐tailed.
Results
Entry characteristics
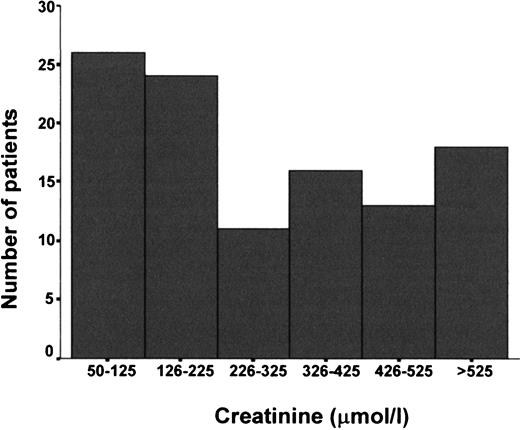
The most frequent initial symptoms related to Wegener's granulomatosis were upper respiratory symptoms in 48% of the patients, pulmonary symptoms in 16%, joint pain in 14% and weight loss, fever and general malaise in 13%. Thirty seven patients (34.3%) also had various degrees of renal involvement at disease presentation. Median time from initial symptoms to inclusion was 6 months (range 0.5–214). Clinical and laboratory data for the 108 patients are depicted in Table 1. Twenty two patients (20.4%) started haemodialysis within 2 weeks of inclusion. ANCA was positive in 95.8% of 97 patients who were tested initially, c‐ANCA was positive in 87.6% and p‐ANCA in 8.2%. One patient was both c‐ and p‐ANCA positive. A 64‐year‐old male patient tested positive for c‐ANCA and anti‐glomerular basement membrane antibodies, but had disease manifestations indistinguishable from Wegener's granulomatosis. A majority of the patients (62.0%) presented with upper respiratory abnormalities, and 51.9% with lower airway involvement (Table 2). The percentage of glomeruli with crescents ranged from 0 to 100% (mean 49.4±32%). In 59% of the patients, more than half of the glomeruli showed extracapillary proliferation (crescents) of varying degrees. In 82.9% of the patients, segmental glomerular necrosis were found. Glomerular filtration rate, as determined by serum creatinine, is shown in Figure 1.
Serum creatinine at inclusion divided into six categories in 108 patients with Wegener's granulomatosis and renal involvement.
Laboratory and clinical data at initial evaluation for 108 patients with Wegener's granulomatosis and renal involvement
| Variable | Results | Reference |
| range | ||
| Male/female | 70/38 | |
| Age years median (range) | 55 (11–80) | |
| B haemoglobin (g/dl)±SD | 10.1±1.8 | 11.5–17.4 |
| B leukocytes (×109/l)±SD | 12.5±4.6 | 3.7–10.0 |
| B thrombocytes (×109/l)±SD | 426±180 | 150–400 |
| ESR (mm/h)±SD | 91±31 | 1–20 |
| CRP (mg/l)±SD | 126±77 | <5 |
| S albumin (g/l)±SD | 30.5±6.5 | 37–48 |
| S creatinine (μmol/l) median (range) | 251 (53–1440) | 60–120 |
| U protein g/24 h median (range) | 1.5 (0–24.8) | <0.200 |
| c‐ANCA n (%)a | 85 (87.6) | |
| p‐ANCA n(%) | 8 (8.2) | |
| Dialysis at inclusion n(%) | 22 (20.4) |
| Variable | Results | Reference |
| range | ||
| Male/female | 70/38 | |
| Age years median (range) | 55 (11–80) | |
| B haemoglobin (g/dl)±SD | 10.1±1.8 | 11.5–17.4 |
| B leukocytes (×109/l)±SD | 12.5±4.6 | 3.7–10.0 |
| B thrombocytes (×109/l)±SD | 426±180 | 150–400 |
| ESR (mm/h)±SD | 91±31 | 1–20 |
| CRP (mg/l)±SD | 126±77 | <5 |
| S albumin (g/l)±SD | 30.5±6.5 | 37–48 |
| S creatinine (μmol/l) median (range) | 251 (53–1440) | 60–120 |
| U protein g/24 h median (range) | 1.5 (0–24.8) | <0.200 |
| c‐ANCA n (%)a | 85 (87.6) | |
| p‐ANCA n(%) | 8 (8.2) | |
| Dialysis at inclusion n(%) | 22 (20.4) |
ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein; c‐ANCA, cytoplasmic antineutrophil cytoplasmic autoantibodies; p‐ANCA, perinuclear antineutrophil cytoplasmic autoantibodies.
aNinety seven patients initially tested for ANCA.
Laboratory and clinical data at initial evaluation for 108 patients with Wegener's granulomatosis and renal involvement
| Variable | Results | Reference |
| range | ||
| Male/female | 70/38 | |
| Age years median (range) | 55 (11–80) | |
| B haemoglobin (g/dl)±SD | 10.1±1.8 | 11.5–17.4 |
| B leukocytes (×109/l)±SD | 12.5±4.6 | 3.7–10.0 |
| B thrombocytes (×109/l)±SD | 426±180 | 150–400 |
| ESR (mm/h)±SD | 91±31 | 1–20 |
| CRP (mg/l)±SD | 126±77 | <5 |
| S albumin (g/l)±SD | 30.5±6.5 | 37–48 |
| S creatinine (μmol/l) median (range) | 251 (53–1440) | 60–120 |
| U protein g/24 h median (range) | 1.5 (0–24.8) | <0.200 |
| c‐ANCA n (%)a | 85 (87.6) | |
| p‐ANCA n(%) | 8 (8.2) | |
| Dialysis at inclusion n(%) | 22 (20.4) |
| Variable | Results | Reference |
| range | ||
| Male/female | 70/38 | |
| Age years median (range) | 55 (11–80) | |
| B haemoglobin (g/dl)±SD | 10.1±1.8 | 11.5–17.4 |
| B leukocytes (×109/l)±SD | 12.5±4.6 | 3.7–10.0 |
| B thrombocytes (×109/l)±SD | 426±180 | 150–400 |
| ESR (mm/h)±SD | 91±31 | 1–20 |
| CRP (mg/l)±SD | 126±77 | <5 |
| S albumin (g/l)±SD | 30.5±6.5 | 37–48 |
| S creatinine (μmol/l) median (range) | 251 (53–1440) | 60–120 |
| U protein g/24 h median (range) | 1.5 (0–24.8) | <0.200 |
| c‐ANCA n (%)a | 85 (87.6) | |
| p‐ANCA n(%) | 8 (8.2) | |
| Dialysis at inclusion n(%) | 22 (20.4) |
ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein; c‐ANCA, cytoplasmic antineutrophil cytoplasmic autoantibodies; p‐ANCA, perinuclear antineutrophil cytoplasmic autoantibodies.
aNinety seven patients initially tested for ANCA.
Major organ system involvement by Wegener's granulomatosis at initial evaluationa
| Organ systems | n (%) |
| Renal | 108 (100) |
| Upper respiratory system | 67 (62.0) |
| Lungs | 56 (51.9) |
| Musculoskeletal system | 44 (40.7) |
| Skin | 21 (19.4) |
| Eye | 20 (18.5) |
| Peripheral nerves | 8 (7.4) |
| Central nervous system | 2 (1.9) |
| Gut | 1 (0.9) |
| Organ systems | n (%) |
| Renal | 108 (100) |
| Upper respiratory system | 67 (62.0) |
| Lungs | 56 (51.9) |
| Musculoskeletal system | 44 (40.7) |
| Skin | 21 (19.4) |
| Eye | 20 (18.5) |
| Peripheral nerves | 8 (7.4) |
| Central nervous system | 2 (1.9) |
| Gut | 1 (0.9) |
aUpper respiratory system, ear, nose, throat. By definition, all patients had renal involvement.
Major organ system involvement by Wegener's granulomatosis at initial evaluationa
| Organ systems | n (%) |
| Renal | 108 (100) |
| Upper respiratory system | 67 (62.0) |
| Lungs | 56 (51.9) |
| Musculoskeletal system | 44 (40.7) |
| Skin | 21 (19.4) |
| Eye | 20 (18.5) |
| Peripheral nerves | 8 (7.4) |
| Central nervous system | 2 (1.9) |
| Gut | 1 (0.9) |
| Organ systems | n (%) |
| Renal | 108 (100) |
| Upper respiratory system | 67 (62.0) |
| Lungs | 56 (51.9) |
| Musculoskeletal system | 44 (40.7) |
| Skin | 21 (19.4) |
| Eye | 20 (18.5) |
| Peripheral nerves | 8 (7.4) |
| Central nervous system | 2 (1.9) |
| Gut | 1 (0.9) |
aUpper respiratory system, ear, nose, throat. By definition, all patients had renal involvement.
Treatment
The initial treatment was i.v. cyclophosphamide in combination with prednisone for 53.7% of the patients, 38.0% were treated with oral cyclophosphamide and prednisone, and 7.4% with prednisone alone or in combination with azathioprine. One patient died before treatment was instituted. Fifty six per cent of the patients were given methylprednisolone in doses between 250 and 1000 mg a day for 1–6 days, and 26.9% were treated with plasmapheresis, the mean number of exchanges being 8.5±5.8.
Disease activity at follow‐up
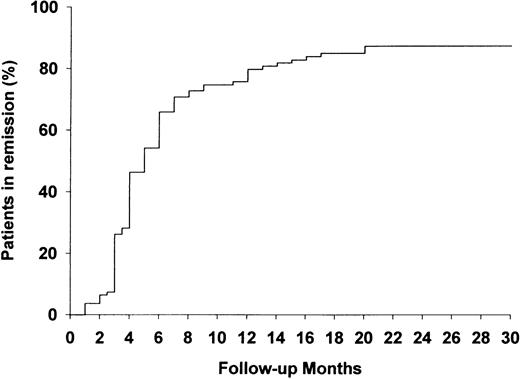
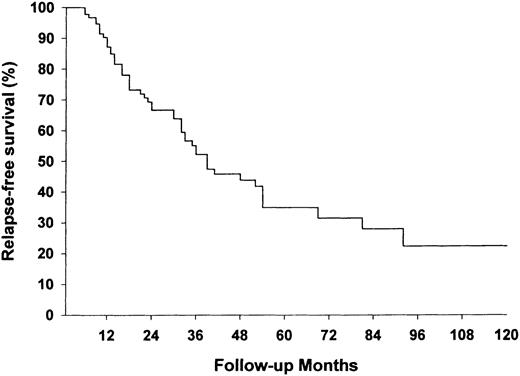
Median follow‐up was 41.5 months (range 0.5–184). The median time to complete remission was 4 months; 88 patients (81.5%) were in remission after 20 months, seven (6.5%) had a partial remission, thus 95 patients (88.0%) showed clinical response. The probability of achieving complete remission is shown in Figure 2. Fifty two patients, 54.7% of those who reached complete or partial remission, relapsed after a median of 22.5 months (range 6–132). The only variable with a significant independent influence on the risk for relapse was the route of cyclophosphamide administration. After adjusting for age, serum creatinine and the number of organs affected, the patients receiving cyclophosphamide in i.v. pulses had a significantly higher risk for relapse than patients treated with oral cyclophosphamide (Table 3). The first relapse occurred in 23 patients (44.2%) while receiving prednisone and cyclophosphamide in tapering doses or with increasing dose interval. Twenty nine patients (55.8%) received no cytotoxic agents, and 10 of them took corticosteroids in daily doses of ≤5 mg. The likelihood of relapse‐free survival is depicted in Figure 3.
The probability of reaching complete remission from Wegener's granulomatosis (%) following induction therapy (n=108).
The probability of relapse‐free survival (%) during follow‐up. Only patients living longer than 3 months were considered to be at risk (n=101).
Relative risk of relapse according to the route of cyclophosphamide administrationa
| Route of administration | Relapse/treatment | Relative risk |
| (months) | (95% CI) | |
| Oral daily | 15/2365 | 1.0 |
| Intravenous pulses | 29/2305 | 2.9 (1.4–5.8) |
| Route of administration | Relapse/treatment | Relative risk |
| (months) | (95% CI) | |
| Oral daily | 15/2365 | 1.0 |
| Intravenous pulses | 29/2305 | 2.9 (1.4–5.8) |
aAdjusted for age, serum creatinine and the number of organs involved at initial evaluation.
Relative risk of relapse according to the route of cyclophosphamide administrationa
| Route of administration | Relapse/treatment | Relative risk |
| (months) | (95% CI) | |
| Oral daily | 15/2365 | 1.0 |
| Intravenous pulses | 29/2305 | 2.9 (1.4–5.8) |
| Route of administration | Relapse/treatment | Relative risk |
| (months) | (95% CI) | |
| Oral daily | 15/2365 | 1.0 |
| Intravenous pulses | 29/2305 | 2.9 (1.4–5.8) |
aAdjusted for age, serum creatinine and the number of organs involved at initial evaluation.
Renal status at follow‐up
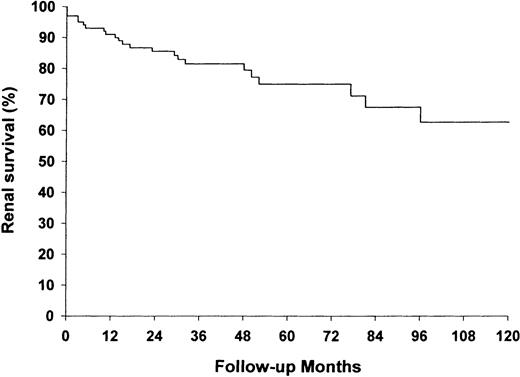
Two and five year renal survival for the total group was 86 and 75%, respectively (Figure 4). Twenty three (22.8%) of the 101 patients who survived the first 3 months developed ESRD after a median of 15 months (range 0–90). Among the 83 patients who were alive at the end of follow‐up, 18.3% were either on dialysis or were transplanted.
Ten (45.5%) of the 22 patients who were on dialysis at inclusion developed ESRD during follow‐up. The corresponding number for those who did not need dialysis at inclusion was 13 (15.1%), and the difference was statistically significant (P=0.001). Twelve (52.2%) of the 23 patients developing ESRD regained renal function and were off dialysis after a mean of 35.8±31.9 days. At the end of follow‐up, four of them were either back on dialysis or were transplanted. The eight patients who did not need to restart dialysis had a mean serum creatinine of 226±110 μmol/l at the end of follow‐up. Using multiple regression analysis, dialysis at inclusion and initial serum creatinine had a significant independent effect on the development of ESRD (Table 4). As expected, patients who reached ESRD received a lower cumulative dose of cyclophosphamide than those who did not, since treatment was often reduced or stopped when ESRD occurred.
There was no difference in renal survival between patients taking oral or intravenous cyclophosphamide, but the median cumulative dose of cyclophosphamide at the end of follow‐up was significantly higher in the group receiving oral than in the group receiving i.v. treatment (48.125 mg vs 17.150 mg P=0.001).
The probability of renal (ESRD‐free) survival (%) during follow‐up. Only patients living longer than 3 months were considered to be at risk (n=101).
Relative risk of developing end‐stage renal disease according to renal functional status at initial evaluationa
| ESRD/treatment | Relative risk | |
| (months) | (95% CI) | |
| S‐creatinine (μmol/l) | ||
| 52–166 | 3/1878 | 1.0 |
| 167–395 | 8/1771 | 2.8 (0.8–10.8) |
| 395–1440 | 12/1836 | 4.1 (1.1–15.1) |
| Dialysis status | ||
| Not dialysis dependent | 13/4502 | 1.0 |
| Dialysis dependent | 10/984 | 3.3 (1.3–8.8) |
| ESRD/treatment | Relative risk | |
| (months) | (95% CI) | |
| S‐creatinine (μmol/l) | ||
| 52–166 | 3/1878 | 1.0 |
| 167–395 | 8/1771 | 2.8 (0.8–10.8) |
| 395–1440 | 12/1836 | 4.1 (1.1–15.1) |
| Dialysis status | ||
| Not dialysis dependent | 13/4502 | 1.0 |
| Dialysis dependent | 10/984 | 3.3 (1.3–8.8) |
aSerum creatinine was divided into three groups with an equal number of patients in each group. Dialysis dependent=dialysis start at ±2 weeks from the initial evaluation.
Relative risk of developing end‐stage renal disease according to renal functional status at initial evaluationa
| ESRD/treatment | Relative risk | |
| (months) | (95% CI) | |
| S‐creatinine (μmol/l) | ||
| 52–166 | 3/1878 | 1.0 |
| 167–395 | 8/1771 | 2.8 (0.8–10.8) |
| 395–1440 | 12/1836 | 4.1 (1.1–15.1) |
| Dialysis status | ||
| Not dialysis dependent | 13/4502 | 1.0 |
| Dialysis dependent | 10/984 | 3.3 (1.3–8.8) |
| ESRD/treatment | Relative risk | |
| (months) | (95% CI) | |
| S‐creatinine (μmol/l) | ||
| 52–166 | 3/1878 | 1.0 |
| 167–395 | 8/1771 | 2.8 (0.8–10.8) |
| 395–1440 | 12/1836 | 4.1 (1.1–15.1) |
| Dialysis status | ||
| Not dialysis dependent | 13/4502 | 1.0 |
| Dialysis dependent | 10/984 | 3.3 (1.3–8.8) |
aSerum creatinine was divided into three groups with an equal number of patients in each group. Dialysis dependent=dialysis start at ±2 weeks from the initial evaluation.
Mortality
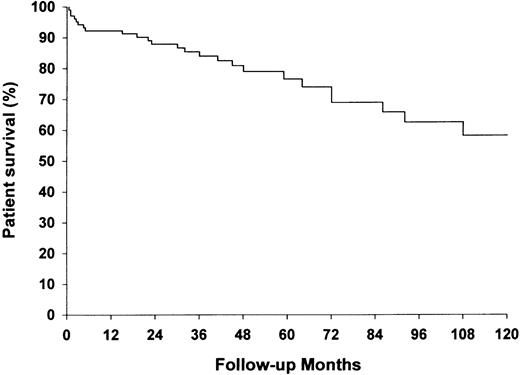
Two‐ and five‐year overall patient survival was 88 and 74%, respectively (Figure 5). Twenty‐six patients (24.1%) had died after a median length of 31 months (range 0.5–169). Seven patients (6.5%) died within the first 3 months. Twelve of the 26 deaths (46.2%) could be related to Wegener's granulomatosis and six (23.1%) to side effects of the treatment (Table 5). Among the 22 patients who were on dialysis at study start, seven (31.8%) died after a median length of 2 months, and four of the seven deaths occurred within 2 months. Serum albumin and age at inclusion showed a significant independent influence on patient mortality. Among patients with serum albumin ≤30 g/l, six of the 12 deaths occurred within 3 months. Age‐adjusted risk of dying according to categories of serum albumin is shown in Table 6. The age‐ and sex‐adjusted MRR for the patient population compared with the general population was 3.8 [95% confidence interval (CI) 2.6–5.6] for both genders combined. The age‐adjusted MRR was 4.0 (95% CI 2.5–6.3) for men and 3.4 (95% CI 1.6–7.2) for women.
Causes of death in 26 patients with Wegener's granulomatosis and renal involvement
| Diagnosis | n |
| Myocardial infarction | 6 |
| Sudden cardiac death | 3 |
| Pneumonia | 3 |
| Pulmonary vasculitis | 3 |
| Pneumocystis carinii pneumonia | 2 |
| Renal failure | 2 |
| Multiorgan failure due to Wegener's granulomatosis | 2 |
| Tracheal stenosis | 1 |
| Gastrointestinal vasculitis | 1 |
| Suicide | 1 |
| Unknown | 2 |
| Diagnosis | n |
| Myocardial infarction | 6 |
| Sudden cardiac death | 3 |
| Pneumonia | 3 |
| Pulmonary vasculitis | 3 |
| Pneumocystis carinii pneumonia | 2 |
| Renal failure | 2 |
| Multiorgan failure due to Wegener's granulomatosis | 2 |
| Tracheal stenosis | 1 |
| Gastrointestinal vasculitis | 1 |
| Suicide | 1 |
| Unknown | 2 |
Causes of death in 26 patients with Wegener's granulomatosis and renal involvement
| Diagnosis | n |
| Myocardial infarction | 6 |
| Sudden cardiac death | 3 |
| Pneumonia | 3 |
| Pulmonary vasculitis | 3 |
| Pneumocystis carinii pneumonia | 2 |
| Renal failure | 2 |
| Multiorgan failure due to Wegener's granulomatosis | 2 |
| Tracheal stenosis | 1 |
| Gastrointestinal vasculitis | 1 |
| Suicide | 1 |
| Unknown | 2 |
| Diagnosis | n |
| Myocardial infarction | 6 |
| Sudden cardiac death | 3 |
| Pneumonia | 3 |
| Pulmonary vasculitis | 3 |
| Pneumocystis carinii pneumonia | 2 |
| Renal failure | 2 |
| Multiorgan failure due to Wegener's granulomatosis | 2 |
| Tracheal stenosis | 1 |
| Gastrointestinal vasculitis | 1 |
| Suicide | 1 |
| Unknown | 2 |
Age‐adjusted relative risk of death according to categories of serum albumin at initial evaluationa
| S‐albumin (g/l) | Deaths/treatment | Relative risk |
| (months) | (95% CI) | |
| 31–52 | 3/1984 | 1.0 |
| 9–30 | 12/1435 | 4.5 (1.3–16.0) |
| S‐albumin (g/l) | Deaths/treatment | Relative risk |
| (months) | (95% CI) | |
| 31–52 | 3/1984 | 1.0 |
| 9–30 | 12/1435 | 4.5 (1.3–16.0) |
an=76. Equal numbers of patients were in each group of serum albumin.
Age‐adjusted relative risk of death according to categories of serum albumin at initial evaluationa
| S‐albumin (g/l) | Deaths/treatment | Relative risk |
| (months) | (95% CI) | |
| 31–52 | 3/1984 | 1.0 |
| 9–30 | 12/1435 | 4.5 (1.3–16.0) |
| S‐albumin (g/l) | Deaths/treatment | Relative risk |
| (months) | (95% CI) | |
| 31–52 | 3/1984 | 1.0 |
| 9–30 | 12/1435 | 4.5 (1.3–16.0) |
an=76. Equal numbers of patients were in each group of serum albumin.
Infections and malignancies
Thirty five of the 108 patients (32.4%) experienced 1–3 episodes of infection requiring hospital admission. Six were caused by Pneumocystis carinii and six by varicella‐zoster virus, both agents known to occur frequently in immunocompromised hosts (Table 7). The risk of serious infection increased with age, and patients 65 years or older had a relative risk of 3.3 (95% CI 1.2–9.0) compared with patients 45 years or younger when adjusted for the given dose of cyclophosphamide and for initial serum creatinine.
Only four patients developed malignant disease during the study period: one squamous and one basal cell carcinoma of the skin, one Kaposi's sarcoma and one gastric carcinoid. There was one event of proven haemorrhagic cystitis in a patient receiving oral cyclophosphamide.
Infectious agents identified in 35 patients with Wegener's granulomatosis and renal involvement during in‐hospital treatment for infection
| Agents | n |
| Pneumocystis carinii | 6 |
| Varicella‐zoster virus | 6 |
| Streptococcus pneumoniae | 5 |
| Straphylococcus aureus | 4 |
| Escherichia coli | 3 |
| Cytomegalovirus | 3 |
| Clostridium difficile | 2 |
| Candida albicans | 1 |
| Herpes simplex | 1 |
| Other Gram‐negative bacteria | 4 |
| Other Gram‐positive bacteria | 3 |
| Agents | n |
| Pneumocystis carinii | 6 |
| Varicella‐zoster virus | 6 |
| Streptococcus pneumoniae | 5 |
| Straphylococcus aureus | 4 |
| Escherichia coli | 3 |
| Cytomegalovirus | 3 |
| Clostridium difficile | 2 |
| Candida albicans | 1 |
| Herpes simplex | 1 |
| Other Gram‐negative bacteria | 4 |
| Other Gram‐positive bacteria | 3 |
Infectious agents identified in 35 patients with Wegener's granulomatosis and renal involvement during in‐hospital treatment for infection
| Agents | n |
| Pneumocystis carinii | 6 |
| Varicella‐zoster virus | 6 |
| Streptococcus pneumoniae | 5 |
| Straphylococcus aureus | 4 |
| Escherichia coli | 3 |
| Cytomegalovirus | 3 |
| Clostridium difficile | 2 |
| Candida albicans | 1 |
| Herpes simplex | 1 |
| Other Gram‐negative bacteria | 4 |
| Other Gram‐positive bacteria | 3 |
| Agents | n |
| Pneumocystis carinii | 6 |
| Varicella‐zoster virus | 6 |
| Streptococcus pneumoniae | 5 |
| Straphylococcus aureus | 4 |
| Escherichia coli | 3 |
| Cytomegalovirus | 3 |
| Clostridium difficile | 2 |
| Candida albicans | 1 |
| Herpes simplex | 1 |
| Other Gram‐negative bacteria | 4 |
| Other Gram‐positive bacteria | 3 |
Discussion
In 108 patients with Wegener's granulomatosis and active renal disease, complete remission was achieved in 81.5% of the patients following induction therapy. The median time to remission was 4 months. Fifty‐five per cent of the patients experienced at least one relapse. The remission rate was somewhat lower than reported in the study by Fauci and co‐workers [2], but comparable with results of studies where only patients with renal disease were included [15,16]. A high proportion of our patients relapsed, and relapses came earlier than reported in some studies [16,17]; however, Jayne and co‐workers found relapses in 41% of their patients after only 1 year of follow‐up [18]. Some studies indicate an increased risk of relapse in small vessel vasculitis where patients have antibodies directed against proteinase 3 (aPR3) rather than against myeloperoxidase (aMPO) [19,20]. The presence of these antibodies was not tested in all our patients, but a majority were c‐ANCA positive, which makes it conceivable that aPR3 was more frequent than aMPA in the present study.
Intravenous cyclophosphamide given in pulses increased the likelihood of relapse compared with daily oral administration after adjusting for age, serum creatinine and for the number of organs affected. The exact treatment schedules varied, but many patients experienced a relapse when the dose interval increased. Intermittent i.v. treatment with cyclophosphamide has been introduced with the intention of reducing side effects from high cumulative doses of the drug, but firm evidence that this can be achieved without compromising remission and relapse rates are lacking. Our results may suggest that fear of side effects could have led to suboptimal cyclophosphamide dose intensity in the i.v.‐treated group. A higher risk for relapse in patients treated with i.v. pulse compared with daily oral cyclophosphamide has also been reported in a recent prospective study [21].
At the time of relapse, the majority of our patients were not receiving cytotoxic agents at all. In the study by Fauci, where only 29% of the patients had relapsed after a mean follow‐up of 51 months, the treatment was more prolonged with a mean duration of therapy of 35 months [2]. These observations can constitute an argument for prolonged therapy including a cytotoxic agent in order to maintain remission. The efficacy of other candidates such as azathioprine and methotrexate has not yet been proven in prospective controlled trials. Interesting results were obtained in an open uncontrolled trial of 42 patients with Wegener's granulomatosis without immediately life‐threatening organ involvement (serum creatinine <221 μmol/l) who received treatment with low dose weekly methotrexate in combination with corticosteroids. Substantial improvement occurred in 83%, but 50% relapsed after a median follow‐up of 2 years [14]. The ongoing multicentre ECSYSVASTRIAL will address some of the questions regarding alternative therapies [22].
Entry serum creatinine was a predictor of renal outcome. Patients with serum creatinine of <170 μmol/l had a 10‐year renal survival of ∼80% (results not shown). A similar result has been found in other studies of patients with vasculitis and renal involvement [15,23,24]. Balow suggested that the risk of ESRD was 10% within 10 years in patients with any sign of renal involvement, and 33% if there was evidence of reduced renal function at the time of diagnosis [25]. Our results are in accordance with this.
Of the patients who initially were on dialysis, 45.5% developed ESRD. Andrassy and co‐workers found that 29% developed ESRD [11], but the follow‐up was shorter than for our patients. In another study of vasculitis where the majority (63%) of the patients were p‐ANCA positive, 78% of those with initial need for dialysis experienced ESRD [16]. Only 74% of the patients received cytotoxic treatment, however, as compared with 100% in the same cohort in our study. It has been suggested that renal lesions in p‐ANCA‐associated glomerulonephritis are more chronic and thus less reversible by treatment than in c‐ANCA‐associated glomerulonephritis [26]. Results from our study and others indicate that a majority of patients on dialysis will profit from treatment and regain a substantial amount of renal function, although usually not total normalization.
The cumulative mortality of 24.1% in our study was 3.8 times higher than expected from the reference population. In a North American study published in 1996, the overall mortality was 4.7 times higher than expected [27], a number comparable with ours. Approximately 70% of the deaths were related to Wegener's granulomatosis or side effects of the treatment. All our patients had renal involvement, and patients with generalized disease are known to have shorter life expectancies than patients with limited disease [9,23,27]. Cardiac disease was the most frequent cause of death, and four of nine cardiac deaths occurred during active Wegener's granulomatosis, which probably contributed directly to the deaths. Cardiac involvement previously has been described in 11.8% [2] and 8% [3] of patients with Wegener's granulomatosis. Pericarditis is the most frequent manifestation, but myocarditis [28] and coronary arteritis [29] have also been reported. A substantial early mortality (27% of all deaths) is an observation also reported by others [17,19,27]. This is in contrast to the lower mortality associated with relapse, probably because most patients at that time are under closer surveillance so that adequate treatment can be instituted early. We found patient survival to be influenced by age and serum albumin. Although there is a correlation between serum albumin and blood haemoglobin, CRP and ESR, none of the other variables had a significant influence on mortality. The reason for the effect of serum albumin on survival could be that it is a parameter influenced both by the degree of systemic inflammatory response and by renal protein loss.
About one‐third of the patients received in‐hospital treatment for a serious infection during the study period, and old patients were at highest risk. Six patients had Pneumocystis carinii pneumonia (PCP) and two of them died. A similar frequency of PCP has been noted in other studies [21], and lymphopenic patients, mainly lacking CD4 T‐cells, seem to be at particular risk [30]. This suggests that prophylactic trimethoprim–sulfamethoxasole should be used during the time of maximum immunosuppression and especially when a cytotoxic agent is combined with high doses of prednisone [31]. In the study by Fauci et al., serious infections were rare, a fact they partly attribute to alternate day prednisone and very close follow‐up to avoid serious leucopenia [2].
The frequency of malignant disease was low in our study, probably because the observation time was relatively short. Others have reported an increased incidence of malignant disease in patients treated for vasculitis [3,15], and the relationship between long‐term use of cyclophosphamide and the development of bladder cancer [32] and leukaemia and pre‐leukaemia [33] is well recognized.
In conclusion, the present study confirms earlier reports that Wegener's granulomatosis is a frequently relapsing disease, and in patients with renal involvement the mortality rate is high and chronic renal failure develops in a considerable fraction of the patients. This observational study indicates that treatment with i.v. pulse cyclophosphamide increases the likelihood of relapse compared with daily oral treatment. A strategy for prolonging remission without inducing serious complications from the treatment seems to be an important objective for further prospective studies.
Correspondence and offprint requests to: Dr Knut Aasarød, Department of Medicine, University Hospital of Trondheim, Olav Kyrres gate 17, N‐7006 Trondheim, Norway.
The following physicians are acknowledged for their generous help in providing clinical information about the patients. T. Apeland, E. Bjørbæk, A. Dale, B. O. Eriksen, H. O. Fadnes, L. Gøransson, O. Herlofsen, O. H. Hunderi, W. Koldingsnes, J. Kronborg, D. Paulsen, C. E. Strømsæther, E. Svarstad and A. B. Tafjord. The authors would also like to thank Øystein Krüger from the Nord‐Trøndelag Health Study for his help with the mortality analysis. The study was supported by grants from the County of Sør‐Trøndelag, the Norwegian Society of Nephrology and Locus of Epidemiology, University of Bergen.
References
Walton EW. Giant cell granuloma of the respiratory tract (Wegener's granulomatosis).
Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years.
Hoffman GS, Kerr GS, Leavitt RY et al. Wegener granulomatosis: an analysis of 158 patients [see comments].
Scott DG, Bacon PA, Elliott PJ, Tribe CR, Wallington TB. Systemic vasculitis in a district general hospital 1972–1980: clinical and laboratory features, classification and prognosis of 80 cases.
Kurland LT, Chuang TY, Hunder G. The epidemiology of systemic arteritis. In: Lawrence RC and Shulman LE, eds.
van der Woude FJ, Rasmussen N, Lobatto S et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis.
Watts RA, Carruthers DM, Scott DG. Epidemiology of systemic vasculitis: changing incidence or definition?
Appel GB, Gee B, Kashgarian M, Hayslett JP. Wegener's granulomatosis—clinical–pathologic correlations and long‐term course.
Luqmani RA, Bacon PA, Beaman M et al. Classical versus non‐renal Wegener's granulomatosis.
Adu D, Howie AJ, Scott DG, Bacon PA, McGonigle RJ, Michael J. Polyarteritis and the kidney.
Andrassy K, Erb A, Koderisch J, Waldherr R, Ritz E. Wegener's granulomatosis with renal involvement: patient survival and correlations between initial renal function, renal histology, therapy and renal outcome.
Falk RJ, Hogan S, Carey TS, Jennette JC. Clinical course of anti‐neutrophil cytoplasmic autoantibody‐associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network [see comments].
Leavitt RY, Fauci AS, Bloch DA et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis.
Sneller MC, Hoffman GS, Talar‐Williams C, Kerr GS, Hallahan CW, Fauci AS. An analysis of forty‐two Wegener's granulomatosis patients treated with methotrexate and prednisone.
Westman KW, Bygren PG, Olsson H, Ranstam J, Wieslander J. Relapse rate, renal survival, and cancer morbidity in patients with Wegener's granulomatosis or microscopic polyangiitis with renal involvement.
Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody‐associated microscopic polyangiitis and glomerulonephritis.
Gordon M, Luqmani RA, Adu D et al. Relapses in patients with a systemic vasculitis.
Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis.
Geffriaud‐Ricouard C, Noel LH, Chauveau D, Houhou S, Grunfeld JP, Lesavre P. Clinical spectrum associated with ANCA of defined antigen specificities in 98 selected patients.
Franssen C, Gans R, Kallenberg C, Hageluken C, Hoorntje S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti‐proteinase 3 and anti‐myeloperoxidase autoantibodies.
Guillevin L, Cordier JF, Lhote F et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis [see comments].
Jayne DR, Rasmussen N. Treatment of antineutrophil cytoplasm autoantibody‐associated systemic vasculitis: initiatives of the European Community Systemic Vasculitis Clinical Trials Study Group.
Briedigkeit L, Kettritz R, Gobel U, Natusch R. Prognostic factors in Wegener's granulomatosis.
Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody‐associated microscopic polyangiitis and glomerulonephritis.
Franssen CF, Gans RO, Arends B et al. Differences between anti‐myeloperoxidase‐ and anti‐proteinase 3‐associated renal disease.
Matteson EL, Gold KN, Bloch DA, Hunder GG. Long‐term survival of patients with Wegener's granulomatosis from the American College of Rheumatology Wegener's Granulomatosis Classification Criteria Cohort.
Goodfield NE, Bhandari S, Plant WD, Morley‐Davies A, Sutherland GR. Cardiac involvement in Wegener's granulomatosis.
Forstot JZ, Overlie PA, Neufeld GK, Harmon CE, Forstot SL. Cardiac complications of Wegener granulomatosis: a case report of complete heart block and review of the literature.
Jarrousse B, Guillevin L, Bindi P et al. Increased risk of Pneumocystis carinii pneumonia in patients with Wegener's granulomatosis [published erratum appears in Clin Exp Rheumatol 1994 Jan–Feb; 12(1): 117].
Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high‐dose corticosteroids for systemic lupus erythematosus [see comments] [published erratum appears in Arthritis Rheum 1997 Sep; 40(9): 1711].
Talar‐Williams C, Hijazi YM, Walther MM et al. Cyclophosphamide‐induced cystitis and bladder cancer in patients with Wegener granulomatosis [see comments].
Pedersen‐Bjergaard J, Ersboll J, Sorensen HM et al. Risk of acute nonlymphocytic leukemia and preleukemia in patients treated with cyclophosphamide for non‐Hodgkin's lymphomas. Comparison with results obtained in patients treated for Hodgkin's disease and ovarian carcinoma with other alkylating agents.





Comments