-
PDF
- Split View
-
Views
-
Cite
Cite
Ming-Tsung Tseng, Song-Chou Hsieh, Chia-Tung Shun, Kuang-Lun Lee, Chun-Liang Pan, Whei-Min Lin, Yea-Hui Lin, Chia-Li Yu, Sung-Tsang Hsieh, Skin denervation and cutaneous vasculitis in systemic lupus erythematosus, Brain, Volume 129, Issue 4, April 2006, Pages 977–985, https://doi.org/10.1093/brain/awl010
Close - Share Icon Share
Abstract
To understand the clinical significance and mechanisms of cutaneous denervation in systemic lupus erythematosus (SLE), we assessed intraepidermal nerve fibre (IENF) density of the distal leg in 45 SLE patients (4 males and 41 females, aged 38.4 ± 13.6 years) and analysed its correlations with pathology, lupus activity, sensory thresholds and electrophysiological parameters. Compared with age- and gender-matched control subjects, SLE patients had lower IENF densities (3.08 ± 2.17 versus 11.27 ± 3.96 fibres/mm, P < 0.0001); IENF densities were reduced in 38 patients (82.2%). Pathologically, 11 patients (24.4%) were found to have definite cutaneous vasculitis; the severity and extent of cutaneous vasculitis were correlated with IENF densities. Patients with active lupus had even lower IENF densities than those with quiescent lupus (1.86 ± 1.37 versus 4.15 ± 2.20 fibres/mm, P = 0.0002). By linear regression analysis, IENF densities were negatively correlated with the SLE disease activity index (r = 0.527, P = 0.0002) and cumulative episodes of lupus flare-up within 2 years before the skin biopsy (r = 0.616, P = 0.0014). Clinically, skin denervation was present not only in the patients with sensory neuropathy but also in the patients with neuropsychiatric syndrome involving the CNS. SLE patients had significantly elevated warm threshold temperatures (P = 0.003) and reduced cold threshold temperatures (P = 0.048); elevated warm threshold temperatures were associated with the reduced IENF densities (P = 0.032). In conclusion, cutaneous vasculitis and lupus activities underlie skin denervation with associated elevation of thermal thresholds as a major manifestation of sensory nerve injury in SLE.
Introduction
Cutaneous tissues are frequently affected among the diverse involvement of organ systems in systemic lupus erythematosus (SLE) (Kotzin, 1996), and the skin is richly innervated by unmyelinated nerve terminals in the epidermis (Kennedy and Wendelschafer-Crabb, 1993). These observations raise the possibility that skin innervation may be susceptible to immunological derangements in SLE. Recently, skin biopsies with the quantification of intraepidermal nerve fibre (IENF) density have become a new approach to investigate the integrity of epidermal innervation (McCarthy et al., 1995; Herrmann et al., 1999; McArthur and Griffin, 2005). IENF densities are significantly reduced in neuropathies involving small-diameter sensory nerves (Kennedy et al., 1996; Holland et al., 1998; Novak et al., 2001; Shun et al., 2004). In SLE, the neurophysiological status deteriorates in a substantial proportion of patients over the disease course (McNicholl et al., 1994; Omdal et al., 2001). A previous study indicated that IENF density was reduced in SLE (Omdal et al., 2002); the significance and pathogenic mechanisms of epidermal denervation in lupus remain elusive.
In addition to assessing epidermal innervation, skin biopsies also provide opportunities to investigate the vasculature of the dermis. Systemic vasculitis may affect the vasa nervorum and epineurial arteries, causing nerve degeneration in vasculitic neuropathy (Younger, 2004; Pagnoux and Guillevin, 2005). In SLE, vasculitis with subsequent axonal degeneration has traditionally been studied by sural nerve biopsies (Hughes et al., 1982; McCombe et al., 1987; Stefurak et al., 1999). Recently, we demonstrated cutaneous vasculitis in the skin with no active vasculitic lesions (Lee et al., 2005). These findings imply that skin biopsies could potentially be useful for examining vascular pathology and exploring the relationship between vasculitis and epidermal denervation in SLE.
Several lines of evidence indicate that epidermal innervation is reduced in inflammatory neuropathies, and this reduction reflects disease activities or severity. IENF densities are reduced in Guillain–Barré syndrome (Pan et al., 2003), chronic inflammatory demyelinating polyneuropathy (Chiang et al., 2002) and anti-myelin-associated glycoprotein neuropathy (Lombardi et al., 2005), and hence associated with functional disabilities and recovery in Guillain–Barré syndrome (Pan et al., 2003). In a typical lupus course, flare-ups of immunological activities result in injury to target organs, leading to irreversible damage, e.g. glomerulonephritis in the kidney (Bernstein et al., 1995). However, it is not clear whether lupus activities influence cutaneous innervation.
To address the issues of skin denervation and its clinical significance in lupus, we studied an SLE cohort by evaluating the extent of cutaneous innervation, and compared that with the results of pathological, immunological, psychophysical and electrophysical studies. IENF densities were reduced in SLE, particularly in patients with cutaneous vasculitis and active lupus. This may provide important information for understanding the pathogenesis of peripheral neuropathies in SLE.
Patients and methods
SLE patients and control subjects
SLE patients were recruited from National Taiwan University Hospital (Taipei, Taiwan) (April 2002 to March 2004). Diagnosis of SLE was based on consensus criteria (Tan et al., 1982) and the neuropsychiatric syndromes were classified according to the standardized definitions (American College of Rheumatology, 1999). Four or more non-neuropsychiatric criteria were required to establish the diagnosis. Patients visited the rheumatologic clinic regularly (S.C.H. and K.L.L.) for laboratory testing, including complements (C3 and C4) and anti-double-stranded DNA (anti-dsDNA) antibodies. The diagnosis of various neuropsychiatric syndromes was based on a review of medical records and neurological examinations (M.T.T. and S.T.H.). To identify neuropsychiatric syndromes based on objective findings, we excluded mild neuropsychiatric symptoms, i.e. headaches, mild depression and anxiety (Ainiala et al., 2001a; Mitsikostas et al., 2004). Patients with clinically suspected involvement of the CNS underwent neuroimaging (CT or MRI), neurophysiological studies (evoked potentials and EEGs) and CSF examinations. In this report, these patients were defined as having CNS neuropsychiatric syndromes.
Peripheral neuropathy was defined according to the neuropathic symptoms or signs. The sensory signs were classified as the small-fibre type with impairment of at least one sensation to pinprick and temperatures and the large-fibre type with impairment of at least one kinaesthetic sensation to joint positioning and vibration (Shun et al., 2004). To avoid confounding symptoms and signs, potential additional neuropathies were not defined in patients with CNS neuropsychiatric syndromes. Patients with conditions known to be associated with peripheral neuropathies were excluded. Initially, 63 consecutive SLE patients were evaluated and 18 of them were excluded: 8 with impaired renal functions, 5 with diabetes, 4 with administration of potentially neurotoxic agents and 1 with alcoholism.
At the time of the skin biopsy, patients were assigned to an active lupus group or a quiescent lupus group. Active lupus or lupus flare-up was defined based on the presence of at least three of the following seven criteria: (i) renal manifestations (proteinuria ≥500 mg/day or urinary casts); (ii) arthritis (≥2 peripheral joints involved); (iii) mucocutaneous manifestations (malar rash, discoid rash or oral ulcers); (iv) serositis (pericarditis, pleurisy or peritonitis); (v) haematological abnormalities (haemolytic anaemia, leucocytes <3500/mm3, lymphocytes <1500/mm3 or platelets <100 000/mm3); (vi) evolving changes in C3 (≥30% reduction in the C3 level); and (vii) evolving changes in anti-dsDNA antibodies (≥50% increase in anti-dsDNA antibodies). These organ syndromes (criteria i–v) had to be newly developed and were defined according to the scale of the European Consensus Lupus Activity Measurement (Vitali et al., 1992). Changes in serological markers (criteria vi and vii) were relative to the last examination. In regularly followed-up SLE patients, baseline values were those obtained at least 1 month before the skin biopsy (Vitali et al., 1992). In newly diagnosed SLE patients, baseline values were those obtained at least 1 month after the skin biopsy. Therefore, changes in serological markers were defined as follows: [(value obtained at the time of skin biopsy − baseline value)/(baseline value)] × 100%. At each visit, disease activity was measured using the commonly used SLE Disease Activity Index (Bombardier et al., 1992). This is based on the presence or absence of 24 most-important descriptors of disease activity, covering the most-frequently affected organ systems in SLE.
Age- and gender-matched control subjects were retrieved from the database, and were carefully evaluated by detailed questionnaires and neurological examinations to exclude anyone with neurological disorders (Chien et al., 2001; Chang et al., 2004; Lin et al., 2005).
Skin biopsy
A skin biopsy was taken following the established procedures after informed consent had been obtained (Chien et al., 2001). The skin surface was anaesthetized with 2% lidocaine, and a punch of 3 mm in diameter was taken from the lateral side of the distal leg, 10 cm above the lateral malleolus. IENF densities are region-dependent (Johansson et al., 1999; Chang et al., 2004). The selection of biopsy site was based on two criteria: (i) the absence of active lupus skin lesions and (ii) the symptomatic side if sensory symptoms were present in only one extremity. All subjects tolerated the procedure with no obvious discomfort. The protocol was approved by the Ethics Committee of National Taiwan University Hospital.
Immunohistochemistry
Skin specimens were fixed with 2% paraformaldehyde–lysine–periodate (PLP) in 0.1 M phosphate-buffered saline, pH 7.4, for 48 h (Hsieh et al., 2000). Sections of 50 µm perpendicular to the dermis were cut on a sliding microtome (model 440E; Microm, Walldorf, Germany). They were quenched with 1% H2O2, blocked with 5% normal goat serum and incubated with rabbit antiserum to protein gene product 9.5 (PGP 9.5; UltraClone, Isle of Wight, UK, diluted 1 : 1000 in 1% normal serum/Tris) at 4°C for 16–24 h. After rinsing in Tris, sections were incubated with biotinylated goat anti-rabbit IgG at room temperature for 1 h, followed by incubation with the avidin–biotin complex (Vector, Burlingame, CA, USA) for another 1 h. The reaction product was demonstrated with chromogen SG (Vector) and counterstained with eosin (Sigma, St Louis, MO, USA).
Quantification of epidermal innervation
Epidermal innervation was quantified by trained examiners who were blinded to the clinical information (Chien et al., 2001). PGP 9.5-immunoreactive nerve fibres in the epidermis were counted at a magnification of ×400 with an Olympus BX40 microscope (Tokyo, Japan) through the depth of the entire section. Each individual nerve with branching points inside the epidermis was counted as one. For epidermal nerves with branching points in the dermis, each individual nerve was counted separately. The length of the epidermis along the upper margin of the stratum corneum in each section was measured using Image-Pro PLUS (Media Cybernetics, Silver Spring, MD, USA). IENF density was therefore derived and expressed as fibres/mm. For each tissue, there were 48–50 sections, and all the sections were sequentially labelled. Every fifth section was immunostained and quantified. The mean value of these sections was considered as IENF density of the tissue specimen. In the distal leg, normative values from our laboratory [mean ± SD (5th percentile)] of IENF density were 11.16 ± 3.70 (5.88) fibres/mm for subjects aged <60 years and 7.64 ± 3.08 (2.50) fibres/mm for subjects aged ≥60 years. The cut-off point for IENF density was 5.88 and 2.50 fibres/mm in the two age groups, respectively (Chang et al., 2004).
Pathological assessment of skin biopsies
The pathological characteristics were examined on formalin-fixed paraffin-embedded skin sections stained with haematoxylin–eosin. Both perivascular inflammation and vascular injury (extravasation of red blood cells, fibrinoid necrosis or disruption of endothelial cell integrity) were required to diagnose definite vasculitis (Collins et al., 2003; Lee et al., 2005). The presence of only one criterion (perivascular inflammation or vascular injury) was defined as borderline vasculitis. The extent of vasculitis was further quantified by calculating the vasculitic ratio (i.e. the number of vessels with vasculitis to the number of total vessels). Five sections with an interval of 50 µm between each section were examined; all cross-sectioned vessels were scored. The involvement of more than one-third of the vessels in the dermis (i.e. vasculitic ratio >0.33) was designated as extensive vasculitis, and the other subgroup as limited vasculitis. Immunohistochemistry was performed using the avidin–biotin–peroxidase complex technique as described above. Cell surface antigens were examined by the following monoclonal antibodies: T cells with CD3 (Ventana Medical System, Tucson, AZ, USA; 1 : 100), B cells with CD20 (DAKO, Glostrup, Denmark; 1 : 100) and macrophages with CD68 (DAKO; 1 : 200) (Hattori et al., 1999; Lee et al., 2005). The skin pathology and phenotypes of cellular infiltration were examined by a pathologist (C.T.S.) in a blinded fashion.
Assessment of sensory thresholds
Quantitative sensory testing (QST) was performed by using a Thermal Sensory Analyser and Vibratory Sensory Analyser (Medoc Advanced Medical System, Minneapolis, MN, USA) to measure sensory thresholds of warm, cold and vibratory sensations following an established protocol (Yarnitsky and Ochoa, 1991; Lin et al., 2005). Briefly, the machine delivered a stimulus of constant intensity which had been determined by the test algorithm. The intensity of the next stimulus was either increased or decreased by a fixed ratio according to the response of the subject, i.e. whether or not the subject perceived the stimulus. Such procedures were repeated until a predetermined difference in the intensity was reached. The mean intensity of the last two stimuli was the threshold for the level method. Thermal thresholds recorded on the toe were expressed as warm threshold temperature and cold threshold temperature. Vibratory thresholds recorded on the lateral malleolus were measured with similar algorithms. These values were compared with normative values for the age, which had been documented previously (Pan et al., 2001, 2003), and are similar to those of the previous reports (Pan et al., 2001, 2003; Shun et al., 2004). The 95th percentile value for warm thresholds and vibratory thresholds and the 5th percentile value for cold thresholds were defined as the cut-off values, and thresholds beyond these values were considered abnormal.
Electrophysiological assessment
Nerve conduction studies (NCS) followed standardized techniques using a Viking IV electromyograph (Nicolet, Madison, WI, USA). An abnormal result on NCS was defined as having abnormalities of one or more nerves with reduced amplitude, prolonged distal latency or slowed nerve conduction velocity. The amplitudes of the compound muscle action potential (CMAP) and sensory action potential (SAP) were compared with the normative data (Pan et al., 2003). We examined bilateral median, ulnar, peroneal, tibial and sural nerves. Sensorimotor polyneuropathy based on NCS was defined as having abnormalities in two or more nerves (Dyck et al., 1985).
Statistical analysis
Numerical variables following a Gaussian distribution were compared using t-test and are expressed as the mean ± SD; for those variables not following a Gaussian distribution, the data are expressed as the median (range) and were analysed with the non-parametric Mann–Whitney U-test. Regression analysis was performed using the statistical software SPSS (SPSS, Chicago, IL, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA). Forward and backward stepwise linear regressions were applied in the multiple linear regression analysis. Results were considered significant if P < 0.05.
Results
Clinical features of SLE patients
There were 45 SLE patients (4 males and 41 females) fulfilling the criteria, with a mean age of 38.4 ± 13.6 years and mean disease duration of 56.8 ± 63.0 months (Table 1). Compared with the quiescent lupus group, patients with active lupus had higher SLE Disease Activity Index (P < 0.0001), shorter SLE durations (P = 0.033), higher levels of anti-dsDNA antibodies (P = 0.018) and lower C3 levels (P = 0.042).
Demographic and laboratory data of SLE patients
. | Active lupus (n = 21) . | Quiescent lupus (n = 24) . | P . | |||
|---|---|---|---|---|---|---|
| Male/female | 4/17 | 0/24 | ||||
| Age (range) (year) | 35.6 ± 14.6 (18–70) | 40.9 ± 12.5 (20–65) | 0.197 | |||
| SLE duration (months) | 35.6 ± 52.0 | 75.4 ± 66.9 | 0.033* | |||
| SLE Disease Activity Index | 14.2 ± 2.9 | 4.6 ± 3.2 | <0.0001* | |||
| Neuropsychiatric syndromes | ||||||
| No | 9 | 16 | 0.140 | |||
| Yes | 12 | 8 | ||||
| With CNS neuropsychiatric syndromes | 9 | 4 | 0.356 | |||
| With peripheral neuropathy | 3 | 4 | ||||
| Blood urea nitrogen (mg/dl) | 17.5 ± 7.4 | 13.7 ± 7.0 | 0.086 | |||
| Creatinine (mg/dl) | 0.73 ± 0.19 | 0.78 ± 0.19 | 0.363 | |||
| Fasting plasma glucose (mg/dl) | 85.0 ± 18.3 | 87.1 ± 14.5 | 0.764 | |||
| Anti-dsDNA (IU/ml) | 193.1 (6.7–733.9) | 38.3 (0.8–555.0) | 0.018* | |||
| C3 (mg/dl) | 41.6 (20.3–134.0) | 73.8 (25.9–117.0) | 0.042* | |||
. | Active lupus (n = 21) . | Quiescent lupus (n = 24) . | P . | |||
|---|---|---|---|---|---|---|
| Male/female | 4/17 | 0/24 | ||||
| Age (range) (year) | 35.6 ± 14.6 (18–70) | 40.9 ± 12.5 (20–65) | 0.197 | |||
| SLE duration (months) | 35.6 ± 52.0 | 75.4 ± 66.9 | 0.033* | |||
| SLE Disease Activity Index | 14.2 ± 2.9 | 4.6 ± 3.2 | <0.0001* | |||
| Neuropsychiatric syndromes | ||||||
| No | 9 | 16 | 0.140 | |||
| Yes | 12 | 8 | ||||
| With CNS neuropsychiatric syndromes | 9 | 4 | 0.356 | |||
| With peripheral neuropathy | 3 | 4 | ||||
| Blood urea nitrogen (mg/dl) | 17.5 ± 7.4 | 13.7 ± 7.0 | 0.086 | |||
| Creatinine (mg/dl) | 0.73 ± 0.19 | 0.78 ± 0.19 | 0.363 | |||
| Fasting plasma glucose (mg/dl) | 85.0 ± 18.3 | 87.1 ± 14.5 | 0.764 | |||
| Anti-dsDNA (IU/ml) | 193.1 (6.7–733.9) | 38.3 (0.8–555.0) | 0.018* | |||
| C3 (mg/dl) | 41.6 (20.3–134.0) | 73.8 (25.9–117.0) | 0.042* | |||
P < 0.05 (t-test).
Demographic and laboratory data of SLE patients
. | Active lupus (n = 21) . | Quiescent lupus (n = 24) . | P . | |||
|---|---|---|---|---|---|---|
| Male/female | 4/17 | 0/24 | ||||
| Age (range) (year) | 35.6 ± 14.6 (18–70) | 40.9 ± 12.5 (20–65) | 0.197 | |||
| SLE duration (months) | 35.6 ± 52.0 | 75.4 ± 66.9 | 0.033* | |||
| SLE Disease Activity Index | 14.2 ± 2.9 | 4.6 ± 3.2 | <0.0001* | |||
| Neuropsychiatric syndromes | ||||||
| No | 9 | 16 | 0.140 | |||
| Yes | 12 | 8 | ||||
| With CNS neuropsychiatric syndromes | 9 | 4 | 0.356 | |||
| With peripheral neuropathy | 3 | 4 | ||||
| Blood urea nitrogen (mg/dl) | 17.5 ± 7.4 | 13.7 ± 7.0 | 0.086 | |||
| Creatinine (mg/dl) | 0.73 ± 0.19 | 0.78 ± 0.19 | 0.363 | |||
| Fasting plasma glucose (mg/dl) | 85.0 ± 18.3 | 87.1 ± 14.5 | 0.764 | |||
| Anti-dsDNA (IU/ml) | 193.1 (6.7–733.9) | 38.3 (0.8–555.0) | 0.018* | |||
| C3 (mg/dl) | 41.6 (20.3–134.0) | 73.8 (25.9–117.0) | 0.042* | |||
. | Active lupus (n = 21) . | Quiescent lupus (n = 24) . | P . | |||
|---|---|---|---|---|---|---|
| Male/female | 4/17 | 0/24 | ||||
| Age (range) (year) | 35.6 ± 14.6 (18–70) | 40.9 ± 12.5 (20–65) | 0.197 | |||
| SLE duration (months) | 35.6 ± 52.0 | 75.4 ± 66.9 | 0.033* | |||
| SLE Disease Activity Index | 14.2 ± 2.9 | 4.6 ± 3.2 | <0.0001* | |||
| Neuropsychiatric syndromes | ||||||
| No | 9 | 16 | 0.140 | |||
| Yes | 12 | 8 | ||||
| With CNS neuropsychiatric syndromes | 9 | 4 | 0.356 | |||
| With peripheral neuropathy | 3 | 4 | ||||
| Blood urea nitrogen (mg/dl) | 17.5 ± 7.4 | 13.7 ± 7.0 | 0.086 | |||
| Creatinine (mg/dl) | 0.73 ± 0.19 | 0.78 ± 0.19 | 0.363 | |||
| Fasting plasma glucose (mg/dl) | 85.0 ± 18.3 | 87.1 ± 14.5 | 0.764 | |||
| Anti-dsDNA (IU/ml) | 193.1 (6.7–733.9) | 38.3 (0.8–555.0) | 0.018* | |||
| C3 (mg/dl) | 41.6 (20.3–134.0) | 73.8 (25.9–117.0) | 0.042* | |||
P < 0.05 (t-test).
Clinically, 20 patients (44.4%) had various neuropsychiatric syndromes. For patients with no CNS neuropsychiatric syndromes, seven had clinical peripheral neuropathy (Table 2); paraesthesia with a glove-stocking distribution was the major presentation, and two had additional neuropathic pain. In summary, six patients had sensory neuropathies (four with pure sensory neuropathy and two with sensory and motor neuropathies) and one patient had pure motor neuropathy.
Clinical findings in SLE patients with peripheral neuropathy
| Patient no . | Age (year/gender) . | Sensory system . | . | Motor system . | . | IENF density (fibres/mm) . | ||
|---|---|---|---|---|---|---|---|---|
. | . | Symptoms . | Signs . | Weakness . | Hyporeflexia . | . | ||
| 1 | 68/M | Acute paraesthesia and pain; symmetric | SF, LF | Distal | Ankle, knee | 1.98 | ||
| 2 | 65/F | Chronic paraesthesia and pain; asymmetric (left > right) | SF | – | Ankle, knee | 2.53 | ||
| 3 | 20/F | Chronic paraesthesia; symmetric | SF | – | – | 0.52 | ||
| 4 | 50/F | Chronic paraesthesia; symmetric | SF | – | – | 2.00 | ||
| 5 | 33/F | – | SF | Proximal and distal | Ankle | 1.38 | ||
| 6 | 22/F | – | SF | – | – | 3.12 | ||
| 7 | 64/F | – | – | Proximal and distal | Ankle, knee | 6.69 | ||
| Patient no . | Age (year/gender) . | Sensory system . | . | Motor system . | . | IENF density (fibres/mm) . | ||
|---|---|---|---|---|---|---|---|---|
. | . | Symptoms . | Signs . | Weakness . | Hyporeflexia . | . | ||
| 1 | 68/M | Acute paraesthesia and pain; symmetric | SF, LF | Distal | Ankle, knee | 1.98 | ||
| 2 | 65/F | Chronic paraesthesia and pain; asymmetric (left > right) | SF | – | Ankle, knee | 2.53 | ||
| 3 | 20/F | Chronic paraesthesia; symmetric | SF | – | – | 0.52 | ||
| 4 | 50/F | Chronic paraesthesia; symmetric | SF | – | – | 2.00 | ||
| 5 | 33/F | – | SF | Proximal and distal | Ankle | 1.38 | ||
| 6 | 22/F | – | SF | – | – | 3.12 | ||
| 7 | 64/F | – | – | Proximal and distal | Ankle, knee | 6.69 | ||
SF, small-fibre sensory sign (impairment of at least one sensation to pinprick and temperatures) and LF, large-fibre sensory sign (impairment of at least one kinaesthetic sensation to joint positioning and vibration).
Clinical findings in SLE patients with peripheral neuropathy
| Patient no . | Age (year/gender) . | Sensory system . | . | Motor system . | . | IENF density (fibres/mm) . | ||
|---|---|---|---|---|---|---|---|---|
. | . | Symptoms . | Signs . | Weakness . | Hyporeflexia . | . | ||
| 1 | 68/M | Acute paraesthesia and pain; symmetric | SF, LF | Distal | Ankle, knee | 1.98 | ||
| 2 | 65/F | Chronic paraesthesia and pain; asymmetric (left > right) | SF | – | Ankle, knee | 2.53 | ||
| 3 | 20/F | Chronic paraesthesia; symmetric | SF | – | – | 0.52 | ||
| 4 | 50/F | Chronic paraesthesia; symmetric | SF | – | – | 2.00 | ||
| 5 | 33/F | – | SF | Proximal and distal | Ankle | 1.38 | ||
| 6 | 22/F | – | SF | – | – | 3.12 | ||
| 7 | 64/F | – | – | Proximal and distal | Ankle, knee | 6.69 | ||
| Patient no . | Age (year/gender) . | Sensory system . | . | Motor system . | . | IENF density (fibres/mm) . | ||
|---|---|---|---|---|---|---|---|---|
. | . | Symptoms . | Signs . | Weakness . | Hyporeflexia . | . | ||
| 1 | 68/M | Acute paraesthesia and pain; symmetric | SF, LF | Distal | Ankle, knee | 1.98 | ||
| 2 | 65/F | Chronic paraesthesia and pain; asymmetric (left > right) | SF | – | Ankle, knee | 2.53 | ||
| 3 | 20/F | Chronic paraesthesia; symmetric | SF | – | – | 0.52 | ||
| 4 | 50/F | Chronic paraesthesia; symmetric | SF | – | – | 2.00 | ||
| 5 | 33/F | – | SF | Proximal and distal | Ankle | 1.38 | ||
| 6 | 22/F | – | SF | – | – | 3.12 | ||
| 7 | 64/F | – | – | Proximal and distal | Ankle, knee | 6.69 | ||
SF, small-fibre sensory sign (impairment of at least one sensation to pinprick and temperatures) and LF, large-fibre sensory sign (impairment of at least one kinaesthetic sensation to joint positioning and vibration).
The other thirteen patients had CNS neuropsychiatric syndromes: six with myelopathy, five with encephalopathy, five with cerebrovascular disorders, four with psychosis, two with seizures and three with mood disorders. Ten patients (22.2%) had more than one neuropsychiatric syndrome. There were no detectable abnormalities on the neurological examinations in the other 25 patients, and they remained free of neuropsychiatric syndromes during follow-up.
Skin innervation in SLE patients
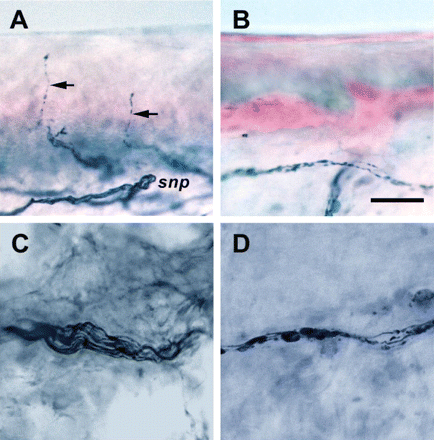
In the leg skin of control subjects, there were abundant PGP 9.5-immunoreactive nerves in the epidermis and dermis. In the epidermis, nerves arose vertically from the subepidermal nerve plexuses with a typical varicose appearance (Fig. 1A). The profiles of individual nerve fibres in dermal nerve bundles were dense with continuous immunoreactivities (Fig. 1C). In contrast, in the skin of SLE patients, epidermal nerves were markedly reduced (Fig. 1B). The immunoreactive pattern of dermal nerve bundles was fragmented, reflecting axonal degeneration (Fig. 1D).
Cutaneous innervation in SLE. Skin sections were immunostained with PGP 9.5. Representative sections are from a control subject (A and C), and from a patient of SLE with peripheral neuropathy (B and D). (A) In normal skin, abundant epidermal nerves (arrows) arise from the subepidermal nerve plexuses (snp). Typical intraepidermal nerve fibres exhibit a wavy course with varicosities. (B) In an SLE patient, the epidermal and dermal nerves are markedly depleted, and the staining pattern of subepidermal nerve plexuses is faint and fragmented. (C) In normal skin, the dermal nerve bundles consist of several axons with dense, linear and continuous PGP 9.5-immunoreactivity. (D) In SLE, the dermal nerve bundles are fragmented with a beaded appearance. Scale bar = 50 µm.
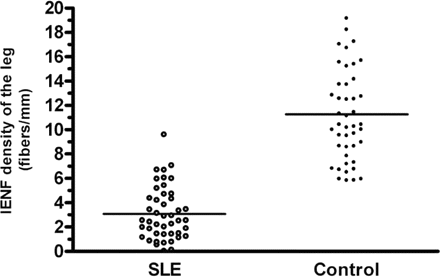
SLE patients had significantly lower IENF densities compared with the age- and gender-matched control subjects (P < 0.0001) (Fig. 2). As a whole, IENF densities were reduced in 38 patients (82.2%).
Reduced IENF densities in SLE. IENF densities of SLE patients (open circles) were markedly reduced compared with those of age- and gender-matched control subjects (closed circles) (3.08 ± 2.17 versus 11.27 ± 3.96 fibres/mm, P < 0.0001). The bars show mean values.
Cutaneous vasculitis and correlation with IENF densities in SLE
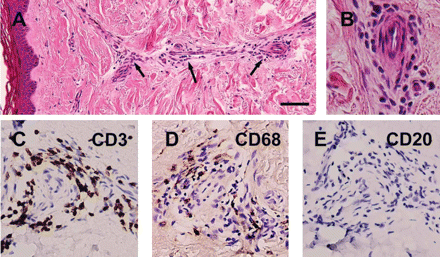
To explore inflammatory vasculopathy, we examined dermal vessels on paraffin-embedded skin sections. Eleven of the forty-five SLE patients (24.4%) had definite vasculitis (Fig. 3A and B); the infiltrating cells were positive for T cells (CD3, Fig. 3C) and macrophages (CD68, Fig. 3D), but negative for B cells (CD20, Fig. 3E).
Cutaneous vasculitis in systemic lupus erythematosus. Paraffin-embedded sections were stained with haematoxylin–eosin (A and B). Adjacent sections were immunostained with markers: CD3 for T cells (C), CD68 for macrophages (D) and CD20 for B cells (E) in brown, and counterstained with haematoxylin. (A and B) Section shows marked perivascular infiltration (arrows) around dermal vessels. (C–E) Inflammatory infiltrate is composed of cells positive for CD3 and CD68, but negative for CD20. Scale bar = 50 µm in (A), 250 µm in (B) and 200 µm in (C–E).
There were grading differences in IENF densities among the three classes of vasculitic pathology; IENF density was the lowest in the group of definite vasculitis. IENF density of the borderline vasculitis group was higher than that of the definite vasculitis group (P = 0.025), but lower than that in the group with no vasculitis (P = 0.048) (Fig. 4). There was no statistical difference in age and gender among the three subgroups.
Association of cutaneous vasculitis with IENF densities in SLE. SLE patients with definite vasculitis (open circles) had significantly lower IENF densities than those with borderline vasculitis (closed circles) [1.45 (0.00–4.70) versus 2.71 (0.75–6.02) fibres/mm, P = 0.025]. IENF densities in patients with borderline vasculitis were lower than those with no vasculitis (squares) [4.61 (0.52–9.58) fibres/mm, P = 0.048]. The bars show median values.
Among the 11 patients in the definite vasculitis group, patients with extensive vasculitis (n = 6) had even lower IENF density than those with limited vasculitis (n = 5) [0.71 (0.00–1.84) versus 2.24 (0.84–4.70) fibres/mm, P = 0.017]. These findings indicated that the severity and extent of cutaneous vasculitis were associated with epidermal denervation.
Correlation of IENF densities with lupus activity
To understand the clinical significance of cutaneous denervation in SLE, we evaluated the association of IENF densities with disease activities. Patients with active lupus had lower IENF densities than those with quiescent lupus (P = 0.0002) (Fig. 5A). Even patients with quiescent lupus had lower IENF densities than those of age- and gender-matched control subjects (4.15 ± 2.20 versus 11.35 ± 4.31 fibres/mm, P < 0.0001).
Correlation of disease activity with IENF density in SLE. (A) IENF densities of patients with active lupus (open circles) were lower than those of patients with quiescent lupus (closed circles) (1.86 ± 1.37 versus 4.15 ± 2.20 fibres/mm, P = 0.0002). (B) IENF densities were negatively correlated with SLE Disease Activity Index (slope = −0.200 ± 0.049, r = 0.527, P = 0.0002). (C) Reduced IENF densities were associated with the presence of neuropsychiatric syndromes (NPS) [1.86 (0.00–6.69) versus 3.82 (0.75–9.58) fibres/mm, P = 0.0011], evolving changes in anti-dsDNA antibodies (anti-dsDNA) [1.87 (0.00–6.68) versus 4.36 (0.10–9.58) fibres/mm, P = 0.012], and evolving changes in C3 (C3) [1.87 (0.00–5.40) versus 3.62 (0.10–9.58) fibres/mm, P = 0.041]. (D) IENF densities were negatively correlated with the cumulative episodes of lupus flare-ups within 2 years before the skin biopsy (slope = −1.218 ± 0.333, r = 0.616, P = 0.0014). In A and C, the bars show mean and median values, respectively. In B and D, the solid lines represent the linear regression lines and dotted lines indicate 95% confidence intervals.
IENF densities were negatively correlated with SLE Disease Activity Index (P = 0.0002) (Fig. 5B). SLE Disease Activity Index remained significantly associated with IENF densities (r = 0.527, P < 0.001) on multiple linear regression analysis including age and gender as independent variables. We further clarified the contribution of different components of European Consensus Lupus Activity Measurement to epidermal denervation; three factors were associated with reduced IENF densities: neuropsychiatric syndromes (P = 0.0011), evolving changes in anti-dsDNA antibodies (P = 0.012) and evolving changes in C3 (P = 0.041) (Fig. 5C).
We tested the hypothesis that the aggravation of lupus activities could influence cutaneous denervation. In the 27 SLE patients with at least 2 years of regular follow-up, IENF densities were negatively correlated with the cumulative episodes of lupus flare-ups within the previous 2 years before skin biopsy (P = 0.0014) (Fig. 5D); this variable remained significant with IENF densities on the model of multiple linear regression (r = 0.616, P = 0.001). IENF densities in patients with more than two episodes of lupus flare-ups (n = 6) were markedly reduced, i.e. less than the first percentile of the normative IENF density (2.50 fibres/mm).
Taken together, lupus activities were associated with reduced IENF densities, and more episodes of lupus flare-ups were correlated with a greater degree of epidermal denervation.
Clinical presentations and skin innervation with SLE
For patients with clinical sensory neuropathies (Patients 1–6 in Table 2), IENF densities were lower than those in the remaining 26 patients with no CNS neuropsychiatric syndromes (P = 0.022) (Table 3). Reduced IENF densities were associated with sensory symptoms (P = 0.043) and small-fibre sensory signs (P = 0.022), but not with motor weakness (P = 0.747) or ankle hyporeflexia (P = 0.498). As described above, the presence of neuropsychiatric syndromes was associated with epidermal denervation (Fig. 5C). Patients with CNS neuropsychiatric syndromes had lower IENF densities than those with no neuropsychiatric syndromes (P = 0.0009); IENF densities were similar between patients with sensory neuropathy and those with CNS neuropsychiatric syndromes (P = 0.592). These findings suggest that cutaneous denervation was not only present in SLE patients with clinical sensory neuropathy, but also existed in patients with CNS neuropsychiatric syndromes.
Clinical presentations and IENF density in SLE patients without CNS neuropsychiatric syndromes (n = 32)
| Parameter . | n . | IENF density . | P . | |||
|---|---|---|---|---|---|---|
| Sensory neuropathy | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Sensory symptoms | ||||||
| Yes | 4 | 1.99 (0.52–2.53) | 0.043* | |||
| No | 28 | 3.63 (0.75–9.58) | ||||
| Small-fibre sensory sign | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Large-fibre sensory sign | ||||||
| Yes | 1 | 1.98 | ||||
| No | 31 | 3.42 (0.52–9.58) | ||||
| Weakness of the foot | ||||||
| Yes | 3 | 1.98 (1.38–6.69) | 0.747 | |||
| No | 29 | 3.42 (0.74–9.58) | ||||
| Hyporeflexia (ankle) | ||||||
| Yes | 4 | 2.26 (1.38–6.69) | 0.498 | |||
| No | 28 | 3.44 (0.75–9.58) | ||||
| Parameter . | n . | IENF density . | P . | |||
|---|---|---|---|---|---|---|
| Sensory neuropathy | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Sensory symptoms | ||||||
| Yes | 4 | 1.99 (0.52–2.53) | 0.043* | |||
| No | 28 | 3.63 (0.75–9.58) | ||||
| Small-fibre sensory sign | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Large-fibre sensory sign | ||||||
| Yes | 1 | 1.98 | ||||
| No | 31 | 3.42 (0.52–9.58) | ||||
| Weakness of the foot | ||||||
| Yes | 3 | 1.98 (1.38–6.69) | 0.747 | |||
| No | 29 | 3.42 (0.74–9.58) | ||||
| Hyporeflexia (ankle) | ||||||
| Yes | 4 | 2.26 (1.38–6.69) | 0.498 | |||
| No | 28 | 3.44 (0.75–9.58) | ||||
IENF density [data are expressed as the median (range)]; sensory neuropathy, defined clinically by symptoms and signs as described in Patients and methods.
P < 0.05 (Mann–Whitney U-test).
Clinical presentations and IENF density in SLE patients without CNS neuropsychiatric syndromes (n = 32)
| Parameter . | n . | IENF density . | P . | |||
|---|---|---|---|---|---|---|
| Sensory neuropathy | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Sensory symptoms | ||||||
| Yes | 4 | 1.99 (0.52–2.53) | 0.043* | |||
| No | 28 | 3.63 (0.75–9.58) | ||||
| Small-fibre sensory sign | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Large-fibre sensory sign | ||||||
| Yes | 1 | 1.98 | ||||
| No | 31 | 3.42 (0.52–9.58) | ||||
| Weakness of the foot | ||||||
| Yes | 3 | 1.98 (1.38–6.69) | 0.747 | |||
| No | 29 | 3.42 (0.74–9.58) | ||||
| Hyporeflexia (ankle) | ||||||
| Yes | 4 | 2.26 (1.38–6.69) | 0.498 | |||
| No | 28 | 3.44 (0.75–9.58) | ||||
| Parameter . | n . | IENF density . | P . | |||
|---|---|---|---|---|---|---|
| Sensory neuropathy | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Sensory symptoms | ||||||
| Yes | 4 | 1.99 (0.52–2.53) | 0.043* | |||
| No | 28 | 3.63 (0.75–9.58) | ||||
| Small-fibre sensory sign | ||||||
| Yes | 6 | 1.99 (0.52–3.12) | 0.022* | |||
| No | 26 | 4.07 (0.75–9.58) | ||||
| Large-fibre sensory sign | ||||||
| Yes | 1 | 1.98 | ||||
| No | 31 | 3.42 (0.52–9.58) | ||||
| Weakness of the foot | ||||||
| Yes | 3 | 1.98 (1.38–6.69) | 0.747 | |||
| No | 29 | 3.42 (0.74–9.58) | ||||
| Hyporeflexia (ankle) | ||||||
| Yes | 4 | 2.26 (1.38–6.69) | 0.498 | |||
| No | 28 | 3.44 (0.75–9.58) | ||||
IENF density [data are expressed as the median (range)]; sensory neuropathy, defined clinically by symptoms and signs as described in Patients and methods.
P < 0.05 (Mann–Whitney U-test).
Sensory thresholds in SLE
Thirty of the thirty-two patients without the CNS involvement agreed to participate in the QST assessment. Compared with control subjects, these 30 SLE patients had significantly higher warm threshold temperatures (39.51 ± 2.17 versus 37.81 ± 2.09°C, P = 0.003) and lower cold threshold temperatures (28.89 ± 1.71 versus 29.59 ± 0.82°C, P = 0.048). Vibratory thresholds did not significantly differ between SLE patients and control subjects (4.13 ± 4.87 versus 2.43 ± 1.23 µm, P = 0.069). Totally, 10 patients (33.3%) had abnormal thresholds: 6 (18.2%) with elevated warm threshold temperature, 4 (13.3%) with reduced cold threshold temperature and 7 (23.3%) with elevated vibratory threshold. IENF densities were associated with abnormal warm thresholds [1.84 (range 0.74–5.40) versus 4.07 (0.86–9.58) fibres/mm, P = 0.032], but not with abnormal cold thresholds (P = 0.12) and vibratory thresholds (P = 0.28).
Electrophysiological studies of SLE
Compared with control subjects, SLE patients had lower amplitudes of sural SAP (15.94 ± 7.99 versus 21.91 ± 7.50 µV, P = 0.0004), peroneal CMAP (3.74 ± 2.31 versus 5.85 ± 1.67 mV, P < 0.0001) and tibial CMAP (13.77 ± 6.16 versus 17.22 ± 4.14 mV, P = 0.002). Of all the 45 patients, 14 (31.1%) had abnormal results on NCS: 7 with sensorimotor polyneuropathy, 4 with polyradiculopathy, 2 with mononeuropathy multiplex and 1 with mononeuropathy; 12 had electrophysiological features of axonal degeneration, while 2 had demyelinating features. Six patients (13.3%) had reduced SAP amplitudes. In these 14 patients with abnormal results on NCS, 4 had clinical sensory neuropathies (Patients 1, 2, 4 and 5 in Table 2), only 1 was free from neuropsychiatric syndromes and the other 9 patients (64.3%) had various CNS neuropsychiatric syndromes: in total 5 with myelopathy, 5 with cerebrovascular disorders, 3 with encephalopathy and 1 with psychosis. Among the 7 patients with clinical peripheral neuropathy only 2 had reduced SAP amplitudes of sural nerves (Patients 2 and 5 in Table 2). Sensory symptoms were not associated with reduced SAP amplitudes (P = 0.825).
Discussion
Two major findings in this report are (i) skin denervation was present not only in lupus patients with sensory neuropathy, but also in patients with CNS neuropsychiatric syndromes; and (ii) the severity and extent of cutaneous vasculitis were strongly associated with skin denervation. In addition, epidermal denervation was correlated with lupus activities. These findings suggest that the reduction in IENF density reflects lupus activity and neural involvement in SLE.
Skin denervation in SLE
The pathological hallmarks of cutaneous nerve degeneration in SLE are similar to those in metabolic and inflammatory sensory neuropathies (McCarthy et al., 1995; Chiang et al., 2002; Pan et al., 2003; Shun et al., 2004). In SLE, the frequency of skin denervation (82.2%) is much higher than those of QST abnormalities (33.3%) and NCS abnormalities (31.1%). Thermal thresholds parallel IENF densities in SLE, similar to diabetic neuropathies (Malik et al., 2001; Shun et al., 2004). The number of patients with clinical sensory neuropathy could be underestimated in the current analysis. The frequent involvement of CNS in SLE might obscure symptoms of peripheral sensory neuropathies (Ainiala et al., 2001b; Brey et al., 2002). For example, all the six patients with overt myelopathy also had reduced IENF densities and, hence, sensory manifestations owing to peripheral nerve injury could not be ascertained in these patients. The concomitant small-fibre sensory neuropathy could only be demonstrated by cutaneous denervation on skin biopsies.
Cutaneous vasculitis and skin denervation
The present report provides direct evidence that cutaneous vasculitis may underlie skin denervation. Traditionally, vasculitic neuropathy was evaluated by sural nerve biopsies (Kissel et al., 1985; Dyck et al., 1987; Mellgren et al., 1989; Collins et al., 2003; Mawrin et al., 2003; Seo et al., 2004). Unmyelinated fibres are vulnerable to vasculitis and ischaemic neuropathy (Parry and Brown, 1982; Vital and Vital, 1985; Lacomis et al., 1997). Patients with cutaneous vasculitis in systemic vasculitides were reported to have a higher prevalence of peripheral neuropathies (Ramos-Casals et al., 2004). However, the functional correlations with unmyelinated fibre pathology are not well characterized. Extending our previous observations on the skin of patients with systemic vasculitic neuropathy (Lee et al., 2005), the current report indicates that vasculitis is present in a significant proportion of SLE patients despite the absence of active lupus lesions. In SLE, the vasculitic pathology could affect sural nerves (McCombe et al., 1987; Bodi et al., 1998; Mawrin et al., 2003). The current study does not exclude the potential involvement of nerve trunks by vasculitis in lupus, but suggests that cutaneous vasculitis could be a manifestation of systemic vasculitis distributed along the nerve trunks as shown by the finding of reduced SAP amplitude in sural nerves. This examination also establishes the application of skin biopsy as a valuable tool in evaluating inflammatory vasculopathy. The correlation of IENF densities with the severity and extent of cutaneous vasculitic pathology not only supports the idea that vasculitis contributes to the pathogenesis of skin denervation, but also extends the previous understanding of sensory neuropathy in vasculitis.
Skin denervation and lupus activities
An intriguing and clinically useful message from the current study is the association of IENF densities with lupus activities. For large-fibre neuropathy in SLE, the associations between electrophysiological parameters and lupus activity are controversial (Omdal et al., 1991; Sivri et al., 1995; Huynh et al., 1999; Omdal et al., 2001; Harel et al., 2002). A critical issue about cutaneous denervation in SLE is how epidermal nerve degeneration evolves during the long disease course of lupus. Cutaneous denervation in SLE did not correlate simply with the disease duration of SLE; instead, IENF densities were correlated with the disease activities and cumulative episodes of lupus flare-ups. Similar to lupus nephritis (Sidiropoulos et al., 2005) and corroborated with a recent study of axonal dysfunction in SLE (Appenzeller et al., 2005), this analysis suggests that aggravation of immune dysregulation on a background of lupus activities precipitates epidermal nerve injury in SLE.
This work was supported by National Taiwan University Hospital, Taipei, Taiwan (NTUH-93S055) and the National Health Research Institute, Taiwan (NHRI-EX94-9323NI).
References
ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes.
Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation.
Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus.
Appenzeller S, Li LM, Costallat LTL, Cendes F. Evidence of reversible axonal dysfunction in systemic lupus erythematosus: a proton MRS study.
Belmont HM, Abramson SB, Lie JT. Pathology and pathogenesis of vascular injury in systemic lupus erythematosus. Interactions of inflammatory cells and activated endothelium.
Bernstein KA, Valerio RD, Lefkowith JB. Glomerular binding activity in MRL lpr serum consists of antibodies that bind to a DNA/histone/type IV collagen complex.
Bodi I, Varadi P, Pokorny G, Engelhardt J, Dibo G, Vecsei L, et al. Polyneuropathy with endoneurial immune complex deposition as the first manifestation of systemic lupus erythematosus.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE.
Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions.
Chang YC, Lin WM, Hsieh ST. Effects of aging on human skin innervation.
Chiang MC, Lin YH, Pan CL, Tseng TJ, Lin WM, Hsieh ST. Cutaneous innervation in chronic inflammatory demyelinating polyneuropathy.
Chien HF, Tseng TJ, Lin WM, Yang CC, Chang YC, Chen RC, et al. Quantitative pathology of cutaneous nerve terminal degeneration in the human skin.
Collins MP, Periquet MI, Mendell JR, Sahenk Z, Nagaraja HN, Kissel JT. Nonsystemic vasculitic neuropathy: insights insights from a clinical cohort.
DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus.
Dyck PJ, Karnes JL, Daube J, O'Brien P, Service FJ. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy.
Dyck PJ, Benstead TJ, Conn DL, Stevens JC, Windebank AJ, Low PA. Nonsystemic vasculitic neuropathy.
Galeazzi M, Annunziata P, Sebastiani GD, Bellisai F, Campanella V, Ferrara GB, et al. Anti-ganglioside antibodies in a large cohort of European patients with systemic lupus erythematosus: clinical, serological, and HLA class II gene associations. European Concerted Action on the Immunogenetics of SLE.
Harel L, Mukamel M, Brik R, Blau H, Straussberg R. Peripheral neuropathy in pediatric systemic lupus erythematosus.
Hattori N, Ichimura M, Nagamatsu M, Li M, Yamamoto K, Kumazawa K, et al. Clinicopathological features of Churg-Strauss syndrome-associated neuropathy.
Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fibre density and sural nerve morphometry in peripheral neuropathies.
Holland NR, Crawford TO, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Small-fibre sensory neuropathies: clinical course and neuropathology of idiopathic cases.
Hsieh ST, Chiang HY, Lin WM. Pathology of nerve terminal degeneration in the skin.
Hughes RA, Cameron JS, Hall SM, Heaton J, Payan J, Teoh R. Multiple mononeuropathy as the initial presentation of systemic lupus erythematosus—nerve biopsy and response to plasma exchange.
Huynh C, Ho SL, Fong KY, Cheung RT, Mok CC, Lau CS. Peripheral neuropathy in systemic lupus erythematosus.
Johansson O, Wang L, Hilliges M, Liang Y. Intraepidermal nerves in human skin: PGP 9.5 immunohistochemistry with special reference to the nerve density in skin from different body regions.
Kennedy WR, Wendelschafer-Crabb G. The innervation of human epidermis.
Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy.
Kissel JT, Slivka AP, Warmolts JR, Mendell JR. The clinical spectrum of necrotizing angiopathy of the peripheral nervous system.
Lacomis D, Giuliani MJ, Steen V, Powell HC. Small fiber neuropathy and vasculitis.
Lee JE, Shun CT, Hsieh SC, Hsieh ST. Skin denervation in vasculitic neuropathy.
Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing.
Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies.
Lombardi R, Erne B, Lauria G, Pareyson D, Borgna M, Morbin M, et al. IgM deposits on skin nerves in anti-myelin-associated glycoprotein neuropathy.
Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, et al. Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology.
Mawrin C, Brunn A, Rocken C, Schroder JM. Peripheral neuropathy in systemic lupus erythematosus: pathomorphological features and distribution pattern of matrix metalloproteinases.
McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy.
McCombe PA, McLeod JG, Pollard JD, Guo YP, Ingall TJ. Peripheral sensorimotor and autonomic neuropathy associated with systemic lupus erythematosus. Clinical, pathological and immunological features.
McNicholl JM, Glynn D, Mongey AB, Hutchinson M, Bresnihan B. A prospective study of neurophysiologic, neurologic and immunologic abnormalities in systemic lupus erythematosus.
Mellgren SI, Conn DL, Stevens JC, Dyck PJ. Peripheral neuropathy in primary Sjöogren's syndrome.
Mitsikostas DD, Sfikakis PP, Goadsby PJ. A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth.
Novak V, Freimer ML, Kissel JT, Sahenk Z, Periquet IM, Nash SM, et al. Autonomic impairment in painful neuropathy.
Omdal R, Henriksen OA, Mellgren SI, Husby G. Peripheral neuropathy in systemic lupus erythematosus.
Omdal R, Loseth S, Torbergsen T, Koldingsnes W, Husby G, Mellgren SI. Peripheral neuropathy in systemic lupus erythematosus—a longitudinal study.
Omdal R, Mellgren SI, Goransson L, Skjesol A, Lindal S, Koldingsnes W, et al. Small nerve fiber involvement in systemic lupus erythematosus: a controlled study.
Pagnoux C, Guillevin L. Peripheral neuropathy in systemic vasculitides.
Pan CL, Lin YH, Lin WM, Tai TY, Hsieh ST. Degeneration of nociceptive nerve terminals in human peripheral neuropathy.
Pan CL, Tseng TJ, Lin YH, Chiang MC, Lin WM, Hsieh ST. Cutaneous innervation in Guillain-Barré syndrome: pathology and clinical correlations.
Parry GJ, Brown MJ. Selective fiber vulnerability in acute ischemic neuropathy.
Ramos-Casals M, Anaya JM, Garcia-Carrasco M, Rosas J, Bove A, Claver G, et al. Cutaneous vasculitis in primary Sjögren syndrome: classification and clinical significance of 52 patients.
Satoi H, Oka N, Kawasaki T, Miyamoto K, Akiguchi I, Kimura J. Mechanisms of tissue injury in vasculitic neuropathies.
Seo JH, Ryan HF, Claussen GC, Thomas TD, Oh SJ. Sensory neuropathy in vasculitis: a clinical, pathologic, and electrophysiologic study.
Sheikh KA, Zhang G, Gong Y, Schnaar RL, Griffin JW. An anti-ganglioside antibody-secreting hybridoma induces neuropathy in mice.
Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, et al. Cutaneous denervation in type 2 diabetes: correlations with diabetic duration and functional impairments.
Sivri A, Hascelik Z, Celiker R, Basgoze O. Early detection of neurological involvement in systemic lupus erythematosus patients.
Spies JM, Westland KW, Bonner JG, Pollard JD. Intraneural activated T cells cause focal breakdown of the blood-nerve barrier.
Stefurak TL, Midroni G, Bilbao JM. Vasculitic polyradiculopathy in systemic lupus erythematosus.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus.
Trysberg E, Tarkowski A. Cerebral inflammation and degeneration in systemic lupus erythematosus.
Vital A, Vital C. Polyarteritis nodosa and peripheral neuropathy: ultrastructural study of 13 cases.
Vitali C, Bencivelli W, Isenberg DA, Smolen JS, Snaith ML, Sciuto M, et al. Disease activity in systemic lupus erythematosus: report of the Consensus Study Group of the European Workshop for Rheumatology. II. Identification of the variables indicative of disease activity and their use in the development of an activity score.
Yarnitsky D, Ochoa JL. Warm and cold specific somatosensory systems. Psychophysical thresholds, reaction times and peripheral conduction velocities.
Author notes
Departments of 1Neurology, 2Internal Medicine and 3Pathology, National Taiwan University Hospital, 4Section of Neurology, Department of Internal Medicine, Far Eastern Memorial Hospital, Departments of 5Forensic Medicine and 6Anatomy and Cell Biology and 7Graduate Institute of Molecular Medicine, National Taiwan University College of Medicine, Taipei, Taiwan and 8Helen Wills Neuroscience Institute, University of California, Berkeley, CA, USA


![Association of cutaneous vasculitis with IENF densities in SLE. SLE patients with definite vasculitis (open circles) had significantly lower IENF densities than those with borderline vasculitis (closed circles) [1.45 (0.00–4.70) versus 2.71 (0.75–6.02) fibres/mm, P = 0.025]. IENF densities in patients with borderline vasculitis were lower than those with no vasculitis (squares) [4.61 (0.52–9.58) fibres/mm, P = 0.048]. The bars show median values.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/129/4/10.1093/brain/awl010/2/m_awl010f4.gif?Expires=1716318732&Signature=rEo3QBggibakAr97mls6EcUNV-8~qcJ2IVU0enJ1uZWJLo8XZBcOedjdEmwXHfmUsfaErHBSIXM7g6XXN2u2O2onW02G4j5loc2twyyJDc9cOFJ6A2fPdABJsxMky9-6nFDnTTbUwVtY6Wh0SLcbCYgJQ05IzwmfkNQ-9DlhLDPICTbtT5WwplL2way1JeprrAMZH7xcLvTTxlTCpLouxiMBbUC77IAdchwJerrGDxVM0KRB-5y33ziuCQQmPWWba~-RFias-6NpwzR83E70gvgJjJD1NenBKu7wSJDr6gKnIhxu9fJFTV1qTzsYeT8KXHFDe2gAUyRusIiBXQYbQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Correlation of disease activity with IENF density in SLE. (A) IENF densities of patients with active lupus (open circles) were lower than those of patients with quiescent lupus (closed circles) (1.86 ± 1.37 versus 4.15 ± 2.20 fibres/mm, P = 0.0002). (B) IENF densities were negatively correlated with SLE Disease Activity Index (slope = −0.200 ± 0.049, r = 0.527, P = 0.0002). (C) Reduced IENF densities were associated with the presence of neuropsychiatric syndromes (NPS) [1.86 (0.00–6.69) versus 3.82 (0.75–9.58) fibres/mm, P = 0.0011], evolving changes in anti-dsDNA antibodies (anti-dsDNA) [1.87 (0.00–6.68) versus 4.36 (0.10–9.58) fibres/mm, P = 0.012], and evolving changes in C3 (C3) [1.87 (0.00–5.40) versus 3.62 (0.10–9.58) fibres/mm, P = 0.041]. (D) IENF densities were negatively correlated with the cumulative episodes of lupus flare-ups within 2 years before the skin biopsy (slope = −1.218 ± 0.333, r = 0.616, P = 0.0014). In A and C, the bars show mean and median values, respectively. In B and D, the solid lines represent the linear regression lines and dotted lines indicate 95% confidence intervals.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/129/4/10.1093/brain/awl010/2/m_awl010f5.gif?Expires=1716318732&Signature=2OVqvnvZY7R4N4tQkG9t00eZ9xARiP2~ak71AHc1sWF4n9a9GcwPs8RB8DGR1nM1Ho~DBTStiEGWfLr9eoPXUliQstIKklCuN~cvgF0D7n6jlyOmzV-b~Q9yhpPmFWIhZZX79t2K2y-7eYOzGjX5eK7QbMfrdS5dRIsnOemuMfEMIs7uMcZ172BuEIh8Jb5WAfTndVuKC9EW1oJlX-KkqR8iaSTKxhaslFirLkfYtQe2SKst3kJudGghfaL9fgL-Kiyt1FbrxFH71wSUng-24w2WG2LNTXjKohs~1a6CQWNmxWDicy7ZgEJdktzn7bhxW3t~4FtJJmcNAYq9555gYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
