-
PDF
- Split View
-
Views
-
Cite
Cite
Curtis L. Cooper, D. William Cameron, Effect of Alcohol Use and Highly Active Antiretroviral Therapy on Plasma Levels of Hepatitis C Virus (HCV) in Patients Coinfected with HIV and HCV, Clinical Infectious Diseases, Volume 41, Issue Supplement_1, July 2005, Pages S105–S109, https://doi.org/10.1086/429506
Close - Share Icon Share
Abstract
Background. The interactions between human immunodeficiency virus (HIV), hepatitis C virus (HCV), alcohol, and antiretroviral therapy are complex.
Methods. We retrospectively assessed persons coinfected with HIV and HCV who achieved HIV suppression to <500 copies/mL and continued to take antiretrovirals for ⩾6 months. Frozen plasma specimens were retrieved for quantitation of HCV RNA levels at baseline and 3, 6, and 12 months after beginning antiretroviral treatment.
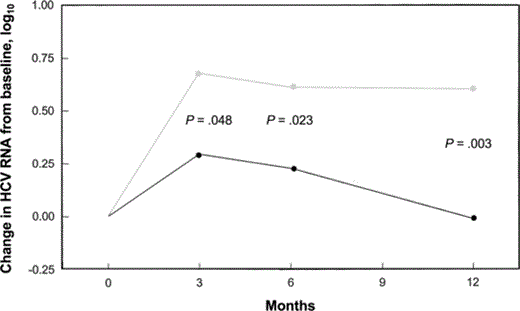
Results. Median HCV RNA levels increased (0.35 log10 IU/mL) at month 3 (n = 44). HCV RNA levels decreased to below baseline by 12 months in patients consuming <50 g of alcohol/day, whereas patients consuming ⩾50 g/day had a sustained increase (>0.6 log10 IU/mL) from baseline (P = .04).
Conclusions. Because low levels of HCV RNA are predictive of a virological response to therapy for HCV infection, it may be advantageous to first achieve suppression of HIV RNA and then initiate treatment for HCV infection in patients coinfected with HIV and HCV. Excess alcohol consumption with therapy for HIV infection increases HCV RNA levels and may impede the effectiveness of this treatment strategy.
The interactions between HAART, immunodeficiency due to HIV, hepatitis C virus (HCV) RNA levels, and consumption of alcohol are complex and not well understood. Although several studies have attempted to describe the changes in HCV RNA levels after the initiation of HAART, no single report clearly describes them from the introduction of therapy to a point when antiretroviral activity is well established and immune reconstitution is equilibrated [1–5]. Many of these investigations are of small size, thereby limiting the ability to draw meaningful conclusions. Almost all results are based on studies of subjects without maximal therapeutic suppression of HIV RNA. Alcohol consumption is not considered.
Methods
Patients coinfected with HIV and HCV who were monitored between January 1996 and August 2001 at the Immunodeficiency Clinic and Viral Hepatitis Clinic at The Ottawa Hospital were identified by retrospective chart review. Subjects who received HAART, continued their therapy for ⩾6 months, and achieved HIV suppression (i.e., <500 copies/mL) were selected. Subjects were excluded if baseline or month 6 quantitative HIV RNA results were not available. No subject received IFN-based therapy for HCV infection.
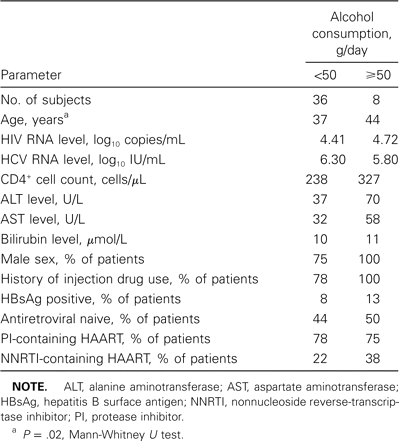
Information pertaining to patients' demographic characteristics (i.e., age and sex), risk factors for HIV and HCV infection, hepatitis B surface antigen status, and alcohol consumption was collected. It was not possible to quantify alcohol consumption by use of a standardized questionnaire, given the retrospective nature of this study. Therefore, alcohol consumption while undergoing HAART was determined from review of chart records. Consumption categories included <50 and ⩾50 g of alcohol/day. The date of initiation of HAART, antiretroviral medications used, and prior exposure to antiretrovirals were recorded. HIV RNA level, CD4+ T lymphocyte count, and alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin levels at baseline and at months 1, 3, 6, and 12 of HAART were collected. Frozen plasma specimens were retrieved for quantification of HCV RNA (Cobas Amplicor HCV Monitor Test, version 2.0; Roche Diagnostic Systems) at baseline and at 3, 6, and 12 months. Subjects with undetectable HCV at all time points were assumed to not be chronically infected and were excluded from analysis.
The difference in log10 HCV RNA level between baseline and each time point was assessed by Wilcoxon signed rank test. The association between clinically relevant variables and change in HCV RNA level from baseline at months 3, 6, and 12 was assessed by Mann-Whitney U test and χ2 test. Analysis was conducted with SPSS, version 11.0. The Ottawa Hospital Research Ethics Board reviewed and approved this study.
Results
Two hundred thirty-six HIV-infected subjects with HCV seropositivity were identified. Of these, 108 (46%) received HAART while being monitored at The Ottawa Hospital Immunodeficiency Clinic. Forty-four patients met the inclusion criteria. Our cohort was characterized by relative preservation of immune function, normal bilirubin levels suggestive of preserved liver function, injection drug use—related infection, and use of protease inhibitor—based HAART (table 1). All 44 patients received HAART for ⩾6 months, and 23 patients received therapy for 12 months. All achieved suppression of HIV RNA (i.e., <500 copies/mL). Thirty-nine achieved HIV RNA levels of <50 copies/mL.
Median baseline characteristics at initiation of HAART among patients coinfected with HIV and hepatitis C virus (HCV), by alcohol consumption.
HCV RNA levels increased by a median of 0.35 log10 (25th and 75th percentiles, -0.04 and 0.57; P < .001) after 3 months of HAART and by 0.30 log10 (25th and 75th percentiles, -0.09 and 0.58; P < .01) after 6 months of HAART. The median log10 HCV RNA level decreased between month 6 and month 12. Alcohol consumption influenced the median change in HCV RNA level from baseline (figure 1). At each time point, the median change in HCV RNA level from baseline was greater in those consuming ⩾50 g of alcohol/day. In subjects not using excess alcohol, HCV RNA levels increased at 3 months after the initiation of therapy but eventually decreased to below baseline after prolonged therapy. Baseline HIV RNA level, CD4+ cell count, age, sex, inclusion of a protease inhibitor in HAART regimen, inclusion of nonnucleoside reverse-transcriptase inhibitors in antiretroviral therapy, and baseline ALT level did not predict change in HCV RNA level between baseline and the other time points measured.
Median change in hepatitis C virus (HCV) RNA levels from baseline in relation to alcohol consumption (gray line, ⩾50 g of alcohol/day; black line, <50 g of alcohol/day). P values were calculated by use of the 2-tailed Mann-Whitney U test.
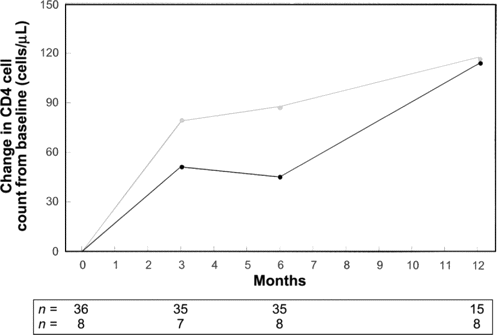
Immune restoration, as determined by increase in median CD4+ T lymphocyte count from baseline, was blunted at month 3 and 6 in those consuming ⩾50 g of alcohol/day (figure 2). This difference was not apparent by month 12 of therapy.
Median change in CD4+ cell count from baseline in relation to alcohol consumption (gray line, ⩾50 g of alcohol/day; black line, <50 g of alcohol/day). P values were calculated by use of the 2-tailed Mann-Whitney U test.
Discussion
Although the relationship between HIV, HCV, and HAART has been evaluated in other investigations, these studies have been characterized by poorly described populations with incomplete suppression of HIV RNA [1–7]. Alcohol consumption also was not considered. As reported elsewhere [1, 6, 7], an initial increase in HCV RNA level from baseline was observed. The pathogenesis is not clear. Endogenous levels of IFN, which are elevated in subjects coinfected with HIV and HCV and correlate with HCV RNA level [8], are reduced after the initiation of HAART [9–11]. Endogenous IFN likely influences the level of HCV RNA [12]. It has been suggested that HAART-induced reduction in these cytokines may result in a transient increase in HCV RNA level [1, 6]. Production of IFN-γ by CD4+ T lymphocytes plays an important role in control and resolution of HCV infection [13, 14]. In fact, CD4+ T lymphocytes of patients ultimately resolving HCV infection produce more IFN-γ after stimulation with HCV antigen than do CD4+ T lymphocytes of patients who do not resolve HCV infection [13, 14]. Improved cytotoxic T lymphocyte (CTL) function with destruction of HCV-infected hepatocytes has been proposed as the mechanism responsible for the initial spike in HCV RNA level observed in some studies [5, 7]. The absence of a substantial increase in transaminase levels in this cohort (data not shown) and in other studies [6] suggests that CTL-mediated hepatocyte destruction is not the mechanism responsible for the transient increase in HCV RNA following initiation of HAART. Direct antiretroviral hepatotoxicity has also been suggested as a mechanism responsible for this initial increase in HCV RNA with HAART [15, 16]. Histological evaluation [7] and the absence of marked transaminitis in the present study and in that of Puoti et al. [6] indicate that other factors, including altered cytokine milieu, also contribute to this phenomenon. It is questionable whether the amount of HCV RNA released from CTL-mediated destruction of HCV-infected hepatocytes or antiretroviral-related hepatotoxicity would be sufficient to account for an increase in HCV RNA level lasting several months.
There are studies suggesting an increase [7, 17–20], decrease [1–3, 21, 22], and absence of change [4] in HCV RNA level from baseline following long-term HAART. Small sample sizes, insufficient suppression of HIV RNA, and the heterogeneity of study cohorts account for much of this disparity. HCV RNA levels declined after month 6 in patients not consuming excess alcohol in this relatively large, well-described cohort with maximal sustained suppression of HIV RNA levels. These results are in agreement with another published evaluation of subjects coinfected with HIV and HCV who achieved “well-controlled viral replication;” however, HIV RNA levels in the subjects evaluated in this subanalysis were not described [22]. Similar to that of HIV-specific immunity [23–28], restoration of the HCV-specific CTL process likely takes many months to occur. Decreasing HCV RNA levels after 6 months of potent HAART may be indicative of this process.
Consumption of ⩾50 g of alcohol/day was a significant predictor of a large increase in HCV RNA levels from baseline (figure 1). A median increase of 0.677 log10 was noted at month 3 in patients drinking alcohol excessively. This elevation from baseline was sustained for at least 12 months after the initiation of HAART. This is likely a result of alcohol-induced hepatocyte toxicity and impaired restoration of HCV-specific cellular immune function. The blunting of CD4+ T lymphocyte recovery (figure 2) supports the latter proposed mechanism. Alcohol has a substantial influence on immunological, virological, and pathological characteristics of chronic HCV infection. In mice, HCV-specific T helper and CTL responses, as well as cytokine expression, are blunted by alcohol consumption [29, 30]. Furthermore, decreased IFN-γ levels, resulting from alcohol-induced dendritic cell dysfunction [30, 31], likely influence HCV RNA levels. Most studies suggest that excess alcohol consumption increases HCV RNA levels [17, 32, 33] and that this relationship is linear [34].
Examination of the effect of alcohol on HCV RNA level may be useful in explaining the initial increase in HCV RNA. If CTL-mediated hepatonecrosis were responsible, then one would predict that the size of the initial increase in HCV RNA levels would be decreased in patients consuming excess alcohol because immune restoration would be blunted. The initial spike in HCV RNA was actually greater in patients consuming excess alcohol. The increase in transaminase levels in patients consuming excess alcohol was modest and similar to that in patients drinking <50 g/day, which suggests that direct, alcohol-induced hepatotoxicity was not occurring.
Because HCV RNA level predicts the likelihood of sustained response to antiviral therapy with IFN and ribavirin [35, 36], these results lend support to the practice of deferring antiviral therapy for HCV until after excess alcohol consumption can be reduced and HAART-induced reduction in HCV RNA level can be achieved. Although this practice is controversial, HIV is often treated before HCV in order to first optimize immune function. It is hypothesized that this may increase the likelihood of achieving sustained virological clearance of HCV with IFN- and ribavirin-based therapy. The blunting of CD4+ T lymphocyte recovery and increase in HCV RNA levels from baseline observed in our study suggest that this strategy may be ineffective if alcohol use is not first curtailed.
Several factors influencing interpretation of these findings should be considered. The results of this study were derived from a selected population of subjects coinfected with HIV and HCV who continued therapy for ⩾6 months and achieved maximal virological suppression. Accurate quantitation of alcohol consumption, even when done prospectively, is difficult. Nevertheless, we are confident that no patients were misclassified on the basis of our detailed chart evaluation. It is possible that alcohol consumption in patients classified as excess users may have varied from month to month during the 12-month course of this study and that this may have influenced the reported virological and immunologic data.
Acknowledgments
Financial support. C.L.C.: Scholarship Award from the Ontario HIV Treatment Network; D.W.C.: Career Scientist Award from the Ontario Ministry of Health/Ontario HIV Treatment Network.
Potential conflicts of interest. C.L.C.: speaker, advisor, and investigator for Abbott, Roche, and Schering Plough; D.W.C.: advisor for Abbott.





Comments