Abstract
BACKGROUND
Neutrophils contribute to the clearance of pathogens through the formation of neutrophil extracellular traps (NETs) in a process known as NETosis, but the excessive release of NETs has been reported to be involved in the pathogenesis of various diseases, including vasculitis, by inducing tissue injury. The aim of the present study was to investigate whether or not NETosis is enhanced in the acute phase of Kawasaki disease (KD).
METHODS
After neutrophils isolated from the peripheral blood of patients with KD and healthy control (HC) were cultured in vitro, the degree of spontaneous NETosis was evaluated by measuring the number of NETs formed and the titers of cell-free DNA (cfDNA) and neutrophil elastase (NE)-DNA complex.
RESULTS
Spontaneous NET formation in vitro was observed in neutrophils isolated from KD patients, and the number of NET formations was significantly higher in acute KD than in convalescent KD and HC. The increased levels of cfDNA and NE-DNA complexes in the acute phase of KD tended to decrease in the convalescent phase.
CONCLUSIONs
Spontaneous NET formation was enhanced in neutrophils from patients with acute KD, suggesting that circulating neutrophils may be primed to undergo NETosis in KD vasculitis.
Similar content being viewed by others
Introduction
Neutrophils are the most abundant leukocytes in the circulation and play a fundamental role in the innate immune response. Neutrophil extracellular traps (NETs) were described for the first time in 2004 by Brinkmann et al.1 NETs are formed via a novel type of active cell death called as NETosis.2 Neutrophils contribute to pathogen clearance by producing NETs, which consist of double-stranded DNA, citrullinated histones, myeloperoxidase (MPO), neutrophil elastase (NE), and antibiotic peptides. Thus, neutrophils can kill microbes intracellularly by phagocytosis and also extracellularly by releasing NETs at inflammatory sites as a biological defense reaction. However, excessive NET formation may trigger tissue injury in diverse conditions of acute and chronic inflammation.3,4 Recent evidence has shown that increased formation or decreased degradation of NETs is associated with clinical manifestations and/or disease activity in sepsis,5,6 systemic lupus erythematosus,7 rheumatoid arthritis,8 and small-vessel vasculitis.9 NETs are also reported to induce endothelial cell damage10,11 and to be involved in the pathogenesis of atherosclerotic progression12,13 and thrombotic complications in coronary ischemic syndromes.14,15
Kawasaki disease (KD) is an acute febrile illness that predominantly affects infants and children.16 Although its etiology remains unknown, KD is well recognized as a type of multisystemic vasculitis associated with coronary artery lesions (CALs).17 This disease leads to the activation and injury of endothelial cells in the acute phase.18 Immunological abnormalities during the acute phase of KD are characterized by a marked activation of the immune system, and high-dose intravenous immunoglobulin (IVIG) is widely used as the standard initial therapy.18,19,20 In the acute phase of KD, the count of circulating neutrophils is elevated in association with a shift to the left. The function of neutrophils in the early stage of KD is reported to be enhanced with a marked increase in reactive oxygen species.21 The plasma levels of NE and MPO were also increased in the acute phase of KD,22 suggesting that the activated neutrophil-mediated endothelial cell injury may be involved in the pathogenesis of KD vasculitis. We previously reported that the apoptosis of neutrophils is inhibited during the acute phase of KD and that the delayed apoptosis of neutrophils may be associated with an increased number of peripheral neutrophils.23 Furthermore, IVIG therapy is reported to decrease the number of circulating neutrophils by accelerating their apoptosis in KD patients.24 Thus, it is suggested that the prolongation of the life span of activated neutrophils in circulation may be involved in the pathogenesis of acute KD. Our search of the literature failed to turn up any reports of NETosis in KD.
The aim of the present study was to investigate whether or not NETosis is enhanced in the acute phase of KD. We evaluated the ability of peripheral neutrophils isolated from KD patients to form NETs in vitro without stimulation by counting the number of NETotic neutrophils via microscopy and measuring the levels of NETosis-derived products (or NET remnants) in the supernatant. We also compared the kinetics of spontaneous NET formation between the acute and convalescent phases of KD.
Materials and methods
Patients and sample collection
We evaluated 37 patients with KD (from 13 to 44 months of age, median age of 25 months) and 6 healthy controls (HC; from 13 to 80 months of age, median age of 16 months). All patients were hospitalized at the National Defense Medical College hospital between August 2016 and April 2018. All KD patients were enrolled within 6 days of the onset of illness, with day 1 defined as the first day of the fever, and all patients met the diagnostic criteria for KD established by the Diagnostic Guidelines for Kawasaki Disease (5th revision).25 All KD patients were treated with oral aspirin (30 mg/kg per day), IVIG (2 g/kg per day), and intravenous ulinastatin (15,000 U/kg in three divided doses).26 No patients with KD had CALs in the present study. HC did not have any underlying diseases and had not received any medication. Informed consent was obtained from parents of patients and HC. These study procedures were approved by the ethics committee of the National Defense Medical College.
Preparation of neutrophils and serum
Serial blood samples were obtained from KD patients in the acute phase, from days 4 to 6 (median day 6), and in the convalescent phase, from days 23 to 28 (median day 25). Neutrophils were isolated from peripheral blood using EasySepTM Direct Human Neutrophil Isolation Kit (STEMCELL Technologies, Vancouver, Canada). Purified neutrophils were resuspended in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA). The viability (≥95%) of the cells was confirmed by a TC-20-automated cell counter (Bio-Rad Laboratories, Hercules, CA, USA). The purity (≥95%) of the cells was confirmed by Wright–Giemsa staining. Neutrophils were cultured with 6% serum obtained from venous blood in 5% CO2 at 37 °C for 3 h.
Immunofluorescence assays and NET counts
Neutrophils (5 × 105 cells) were transferred to 24-well flat-bottom plates, in which poly-lysine-treated coverslips were placed (Matsunami, Osaka, Japan). After 4-h incubation in 5% CO2 at 37 °C, the neutrophils were fixed with paraformaldehyde (3%) and treated by 0.5% TritonTM X-100 (Sigma-Aldrich, Darmstadt, Germany). Neutrophils were stained with a mouse anti-MPO monoclonal antibody (mAb) (clone ab25989; Abcam, Cambridge, UK) and a rabbit anti-NE mAb (clone ab68672; Abcam). A goat anti-mouse Alexa Fluor® 488 (clone ab150077; Abcam) and anti-rabbit Alexa Fluor® 555 (clone ab150114; Abcam) were utilized as secondary antibodies. DNA was stained with Hoechst 33342 (PromoKine, Heidelberg, Germany). Visualization was performed via fluorescence microscopy using a BZ-X710 microscope (Keyence, Osaka, Japan) equipped with ×20 objective lens (CFI Plan Apochromat Lambda ×20; Nikon, Tokyo, Japan). To measure the number of NETs formed, the microscopic fields were subdivided into 682 fields for the whole coverslip area. The number of NETs formed was defined as the number of fields in which NETs were observed.
Quantification of cfDNA in the supernatant
Neutrophils (1 × 106 cells) were transferred to 24-well flat-bottom plates without coverslips. After 3-h incubation in 5% CO2 at 37 °C, EDTA (10 mM) was added to the supernatant. The supernatant was obtained by centrifugation at 16,000 × g for 10 min and purified by Maxwell® Rapid Sample Concentrator (AS1480; Promega, Madison, WI, USA). The concentration of cell-free DNA (cfDNA) was determined by using an InvitrogenTM QubitTM 3 Fluorometer (Thermo Fisher Scientific). The number of copies of the ribonuclease P (RNaseP), which was used as a housekeeping gene, was measured using real-time polymerase chain reaction (PCR; LightCycler® 480 system II) with TaqManTM RNaseP primer Mix (Roche, Basel, Switzerland). The value of RNaseP was expressed as copies/μg DNA.
Measurement of NE-DNA complexes
Neutrophils (1 × 106 cells) were transferred to 24-well flat-bottom plates with coverslips. After 3-h incubation in 5% CO2 at 37 °C, NE-DNA complexes were measured using a NETosis Assay Kit (Cayman Chemical, Ann Arbor, MI, USA). In brief, unbound NE in the supernatant was washed away following NET generation. Soluble elastase was dissociated from NET-associated DNA by adding S7 nuclease and was then added to a substrate, which was selectively cleaved by elastase to yield a 4-nitroaniline product that absorbs light at 405 nm.
Measurement of cfDNA in serum samples
Serial blood samples were obtained from KD patients in the acute and convalescent phases and from HC. All serum samples were stored at −80 °C until they were analyzed. The serum levels of cfDNA were measured using a Cell Death Detection ELISAPLUS (Roche, Mannheim, Germany).
Statistical analyses
All of the data were presented as the median (25th–75th percentiles) for continuous variables or as percentages for categorical variables. The statistical analyses were performed with GraphPad PRISM version 6.07 (GraphPad Software, San Diego, CA, USA). Any differences among the acute and convalescent phases in the KD group were assessed by Wilcoxon’s signed-rank test. Intergroup differences among the KD patients and HC were analyzed using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. In all statistical analyses, P < 0.05 was considered to be statistically significant.
Results
Subjects’ characteristics and laboratory findings
The clinical and laboratory data were compared between the KD and HC groups (Table 1). The acute KD group tended to have significantly higher values for white blood cells (WBCs), neutrophils, and C-reactive protein (CRP) and significantly lower values for lymphocytes, albumin, and sodium than the convalescent KD and HC groups (P < 0.01). The acute KD group also tended to have significantly higher levels of total bilirubin and alanine aminotransferase (ALT) than the convalescent KD group (P < 0.01).
Spontaneous NET formation by unstimulated neutrophils in vitro
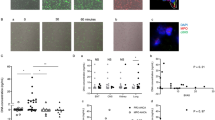
After neutrophils isolated from patients in the acute and convalescent KD and HC were incubated for 4 h in 5% CO2 at 37 °C, the cells were fixed and stained with Hoechst/DNA, NE, and MPO. Spontaneous NET formations were observed by immunofluorescence microscopy, and the representative data are shown in Fig. 1. NET formations were observed in the unstimulated neutrophils from acute KD patients, but not in those from convalescent KD patients and HC.
After neutrophils (5 × 105 cells) isolated from patients in acute (a) and convalescent (b) KD and healthy controls (c) were incubated for 4 h in 5% CO2 at 37 °C, the cells were fixed and stained with Hoechst/DNA (white), neutrophil elastase (NE, green), and myeloperoxidase (MPO, red). Spontaneous NET formation was visualized by immunofluorescence microscopy using a ×200 objective. Scale bars, 50 μm.
The amount of spontaneous NET formation
The number of NET formation was determined by a microscopic observation (Fig. 2). The NET formation counts were significantly higher (P < 0.01) in the acute phase of KD than in the convalescent phase of KD and HC (Fig. 2a). The time-course changes of NET counts in the 11 KD patients whose data could be consecutively acquired in both the acute and convalescent phases are shown in Fig. 2b. The increased NET counts in the acute phase of KD significantly decreased in the convalescent phase (P < 0.01).
a The number of NETs formed was determined by a microscopic observation in acute KD, convalescent KD, and HC, and b the time-course changes in the NET counts between acute and convalescent KD in the 11 KD patients is shown. *P < 0.01 versus convalescent KD and HC using the Mann–Whitney U test. **P < 0.01 versus convalescent KD using Wilcoxon’s signed-rank test.
The titers of cfDNA in the supernatants
The titers of cfDNA released from neutrophils into the supernatants were determined using quantitative PCR (Fig. 3). There were no significant differences among the three groups (Fig. 3a). The time-course changes in the cfDNA titer in the 14 KD patients whose data could be consecutively acquired in both the acute and convalescent phases are shown in Fig. 3b. The increased titers of cfDNA in the acute phase of KD significantly decreased in the convalescent phase (P < 0.01).
The levels of NE-DNA complexes produced by NETosis
The levels of NE-DNA complexes produced by NETotic neutrophils were measured using a NETosis Assay Kit (Fig. 4). The levels of NE-DNA complexes were significantly higher in the acute phase of KD than in the convalescent phase (P < 0.01) and in HC (P < 0.05) (Fig. 4a). The time-course changes in NE-DNA complex levels in the 15 KD patients whose data could be consecutively acquired in both the acute and convalescent phases are shown in Fig. 4b. The increased levels of NE-DNA complexes in the acute phase of KD significantly decreased in the convalescent phase (P < 0.05). Although two KD patients showed an increase in NE-DNA levels from the acute to the convalescent phases, they responded to IVIG therapy and had no specific findings in the clinical course.
a The levels of NE-DNA complexes were measured using an assay kit in acute KD, convalescent KD, and HC, and b the time-course changes in the NE-DNA levels between acute and convalescent KD in the 15 KD patients is shown. *P < 0.01 versus convalescent KD and *P < 0.05 versus HC using the Mann–Whitney U test. **P < 0.05 versus convalescent KD using Wilcoxon’s signed-rank test.
The levels of cfDNA in serum samples
The serum levels of cfDNA were measured using an ELISA (enzyme-linked immunosorbent assay) in 15 KD patients whose data could be consecutively acquired in both the acute and convalescent phases and in six HCs (Fig. 5). The levels of cfDNA were significantly higher (P < 0.01) in the acute phase of KD than in the convalescent phase or HC (Fig. 5a). Regarding the time-course changes, the increased levels of cfDNA in the acute phase of KD significantly decreased in the convalescent phase (P < 0.01) (Fig. 5b). When each cfDNA titer was corrected by the count of circulating neutrophils, there were no significant differences among the three groups (Fig. 5c) or between the acute and convalescent phases of KD (Fig. 5d).
a The levels of cfDNA were measured using an ELISA Kit in acute KD, convalescent KD, and HC, and b the time-course changes in the cfDNA levels between acute and convalescent KD in the 15 KD patients is shown. Each cfDNA titer was corrected by the count of circulating neutrophils (c, d). *P < 0.01 versus convalescent KD and HC using the Mann–Whitney U test. **P < 0.01 versus convalescent KD using Wilcoxon’s signed-rank test.
Discussion
In the present study, we showed that spontaneous NET formations in vitro were observed in neutrophils isolated from KD patients, and that the amount of NET formations was significantly higher in acute KD than in convalescent KD and HC. The increased levels of cfDNA and NE-DNA complexes in the acute phase of KD tended to decrease in the convalescent phase of KD. These results indicate that the neutrophils isolated from the acute KD are more susceptible to NETosis than those from convalescent KD patients and HC.
NETosis is a recently described mechanism of neutrophil death that occurs upon neutrophil activation, ultimately leading to the release of NETs.1,2 NET formation is an essential part of the innate immune response to microbe infections, but spontaneous NET formation of neutrophils isolated from patients with several diseases is reported to be enhanced in vitro. Neutrophils from patients with autoimmune small-vessel vasculitis have shown enhanced spontaneous NETosis in vitro.9,27 Neutrophils from patients with rheumatoid arthritis also exhibit increased spontaneous NET formation in vitro, associated with the enhanced expression of NE and MPO.8 Spontaneous NET formation in vitro is observed in septic or burn patients, but not in healthy donors.28 To our knowledge, this is the first report showing that the spontaneous NET formation was enhanced in the acute phase of KD. However, the mechanism of enhanced NETosis in KD patients is still unclear. Proinflammatory mediators, including tumor necrosis factor-α, interferon-γ, and interleukin-8 and -17, are reported to induce neutrophils to form NETs.29 KD patients have high levels of these proinflammatory cytokines,18,19 which might prime neutrophils for NETosis in circulation.
NETs are composed of DNA, histones, and antimicrobial proteins, which are released extracellularly through NETosis. In the present study, we determined the titers of cfDNA released from NETotic neutrophils in vitro into the supernatant using quantitative PCR (Fig. 3), as in a previous report.8 Because the measurement unit of cfDNA is ×106, the measured values might seem to overlap between groups (Fig. 3). Because the number of neutrophils was insufficiently determined in 1 HC (13 months old), the titers of cfDNA were measured using real-time PCR in five out of six HCs. In addition, the serum cfDNA levels were measured using an ELISA (Fig. 5). The levels of cfDNA were significantly higher in the acute phase of KD than in the convalescent phase or in the HC, while the levels of cfDNA corrected by circulating neutrophil counts showed no significant differences among the groups. Circulating cfDNA is not a specific marker for NETosis, because it is also released from certain cells by apoptosis and necrosis. Circulating cfDNA is reported to increase in coronary artery disease, sepsis, systemic lupus erythematosus, severe burn injury, and cancers.30,31,32,33,34,35 To determine the relevance of circulating cfDNA to the pathogenesis of KD, further studies will be needed. In the present study, we measured the levels of NE-DNA complexes as a specific marker for NETosis-derived products. The levels of NE-DNA complexes increased in neutrophils from the acute phase of KD and tended to decrease in those from the convalescent phase (Fig. 4). Furthermore, these results nearly match the kinetics of the number of NET formations observed on microscopy (Fig. 2). These findings indicate that the NET formation in neutrophils from acute KD patients is enhanced compared with that in convalescent KD patients and HC.
The role of NETosis in the pathophysiology of various diseases remains a matter of debate, but excessive production of NETs has been reported to induce organ injury and failure.3,4 NETosis is correlated with the risk of thromboembolism and disseminated intravascular coagulation in sepsis,5,6 the disease activity of systemic lupus erythematosus,7,36 and the generation of auto-antigens in rheumatoid arthritis.8 The present results show that the circulating neutrophils in the acute phase of KD are susceptible to NETosis. NE and MPO are important component of NETs,1 and KD patients have elevated plasma levels of NE and MPO in the acute phase,22 suggesting that enhanced NET formation may contribute to the pathogenesis of KD vasculitis. However, there is no conclusive proof that this enhanced NETosis leads to endothelial cell injury in KD. NETosis might be a biological phenomenon for capturing unknown pathogens associated with this disease. While netting neutrophils produce a variety of proinflammatory mediators, aggregated NETs are reported to promote the resolution of neutrophil-induced inflammation by degrading cytokines and chemokines via proteases in NETs.37 Thus, NETs have a bilateral character,38 and the physiological and pathological significance of NETosis warrants further investigation.
Several limitations associated with the present study warrant mention. First, because we were unable to prove the in vivo NET formation in patients with KD, there is no direct evidence that NETosis is involved in the pathogenesis of KD vasculitis. However, it is difficult to obtain histological materials from KD patients. It might be worth investigating whether or not NET formation is detected in vivo in a KD-like mouse model. Second, we did not investigate the spontaneous NET formation in the disease control group because such an analysis in children is not ethically permissible. Therefore, whether or not the enhanced NETosis in acute KD is a specific finding in pediatric infectious diseases remains unclear. Third, since there were no KD patients with CALs in the present study, we could not compare the degree of NET formation between KD patients with and without CALs. Therefore, whether or not increased NET formation is associated with CAL formation in KD vasculitis is unclear. To determine the relationship between the degree of NETosis and CAL formation in KD, a larger study will be needed in the future.
In conclusion, spontaneous NET formations were enhanced in neutrophils from the acute phase of KD. The increased degree of spontaneous NET formation in acute KD tended to decrease in convalescent KD. These results indicate that circulating neutrophils are primed to undergo NETosis in the acute phase of KD, suggesting the possible involvement of NETosis in the pathogenesis of KD vasculitis. However, we were unable to determine the pathophysiological relevance of NETosis for arthritis in the acute phase of KD. Direct evidence that NETosis is involved in the pathogenesis of KD will need to be gathered in a future study.
References
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 (2007).
Kim, S. J. & Jenne, C. N. Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin. Immunol. 28, 546–554 (2016).
Kruger, P. et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 11, e1004651 (2015).
Yang, S. et al. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock 47, 132–139 (2017).
Delabranche, X. et al. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. J. Shock 47, 313–317 (2017).
Leffler, J. et al. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res. Ther. 15, R84 (2013).
Sur Chowdhury, C. et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 16, R122 (2014).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7, e32366 (2012).
Megens, R. T. et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 107, 597–598 (2012).
Warnatsch, A. et al. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320 (2015).
de Boer, O. J. et al. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb. Haemost. 109, 290–297 (2013).
Pertiwi, K. R. et al. Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb. Haemost. 118, 1078–1087 (2018).
Kawasaki, T. et al. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 54, 271–276 (1974).
Kato, H. et al. Coronary aneurysms in infants and young children with acute febrile mucocutaneous lymph node syndrome. J. Pediatr. 86, 892–898 (1975).
Leung, D. Y. et al. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 2, 1298–1302 (1989).
Burns, J. C. & Glode, M. P. Kawasaki syndrome. Lancet. 364, 533–544 (2004).
Burns, J. C. & Franco, A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev. Clin. Immunol. 11, 819–825 (2015).
Niwa, Y. & Sohmiya, K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J. Pediatr. 104, 56–60 (1984).
Takeshita, S. et al. The role of bacterial lipopolysaccharide-bound neutrophils in the pathogenesis of Kawasaki disease. J. Infect. Dis. 179, 508–512 (1999).
Tsujimoto, H. et al. Delayed apoptosis of circulating neutrophils in Kawasaki disease. Clin. Exp. Immunol. 126, 355–364 (2001).
Tsujimoto, H. et al. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin. Immunol. 103, 161–168 (2002).
Ayusawa, M. et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Kawasaki Disease Research Committee. Pediatr. Int. 47, 232–234 (2005).
Kanai, T. et al. Ulinastatin, a urinary trypsin inhibitor, for the initial treatment of patients with Kawasaki disease: a retrospective study. Circulation 124, 2822–2828 (2011).
Söderberg, D. et al. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford) 54, 2085–2094 (2015).
Kaufman, T. et al. Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clin. Immunol. 183, 254–262 (2017).
Keshari, R. S. et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE 7, e48111 (2012).
Borissoff, J. I. et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler. Thromb. Vasc. Biol. 33, 2032–2040 (2013).
Rhodes, A. et al. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit. Care 10, R60 (2006).
Dwivedi, D. J. et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Canadian Critical Care Translational Biology Group. Crit. Care 16, R151 (2012).
Zhang, S. et al. Elevated plasma cfDNA may be associated with active lupus nephritis and partially attributed to abnormal regulation of neutrophil extracellular traps (NETs) in patients with systemic lupus erythematosus. Intern. Med. 53, 2763–2771 (2014).
Altrichter, J. et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur. J. Trauma Emerg. Surg. 36, 551–557 (2010).
Aarthy, R. et al. Role of circulating cell-free DNA in cancers. Mol. Diagn. Ther. 19, 339–350 (2015).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Schauer, C. et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517 (2014).
Kaplan, M. J. & Radic, M. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189, 2689–2695 (2012).
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (#16K10082) in Japan.
Author information
Authors and Affiliations
Contributions
S.T. wrote the first draft of the present manuscript. Y.Y. designed the present work. Y.K. acquired the data from medical records. T.K. and Y.T. analyzed and interpreted the data. S.N. revised the present work critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoshida, Y., Takeshita, S., Kawamura, Y. et al. Enhanced formation of neutrophil extracellular traps in Kawasaki disease. Pediatr Res 87, 998–1004 (2020). https://doi.org/10.1038/s41390-019-0710-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0710-3
This article is cited by
-
Innate immune dysregulation in multisystem inflammatory syndrome in children (MIS-C)
Scientific Reports (2023)
-
Inflammation and aging: signaling pathways and intervention therapies
Signal Transduction and Targeted Therapy (2023)
-
Cerebrovascular involvement in systemic childhood vasculitides
Clinical Rheumatology (2023)
-
Neutrophil degranulation and severely impaired extracellular trap formation at the basis of susceptibility to infections of hemodialysis patients
BMC Medicine (2022)
-
Does the newly observed inflammatory syndrome in children demonstrate a link between uncontrolled neutrophil extracellular traps formation and COVID-19?
Pediatric Research (2021)