Key Points
-
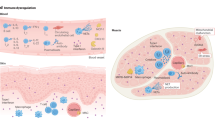
Myositis-specific autoantibodies (MSAs) are present in the majority of juvenile and adult cases of idiopathic inflammatory myopathy and are largely mutually exclusive.
-
Anti-TIF1γ, anti-NXP2 and anti-MDA5 antibodies are present in just over half of juvenile dermatomyositis cases, where they identify distinct subsets of disease.
-
In adults, anti-TIF1γ antibodies are associated with myositis-associated cancer, anti-HMGCR antibodies with statin-induced myosotis and anti-cN1A antibodies with inclusion body myositis.
-
The presence of anti-MDA5 antibodies in either myositis or clinically amyopathic dermatomyositis is a risk factor for rapidly progressive interstitial lung disease, particularly in Eastern Asian populations.
-
Interstitial lung disease is a prominent manifestation of antisynthetase syndrome, which is defined by the presence of antibodies to certain amino-acyl transfer RNA synthetases.
-
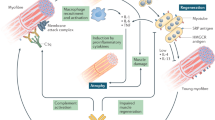
The discovery of novel myositis autoantigens is providing insights into pathogenesis and the links between autoimmunity and cancer.
Abstract
The discovery of novel autoantigen systems related to idiopathic inflammatory myopathies (collectively referred to as myositis) in adults and children has had major implications for the diagnosis and management of this group of diseases across a wide range of medical specialties. Traditionally, autoantibodies found in patients with myositis are described as being myositis-specific autoantibodies (MSAs) or myositis-associated autoantibodies (MAAs), depending on their prevalence in other, related conditions. However, certain MSAs are more closely associated with extramuscular manifestations, such as skin and lung disease, than with myositis itself. It is very rare for more than one MSA to coexist in the same individual, underpinning the potential to use MSAs to precisely define genetic and disease endotypes. Each MSA is associated with a distinctive pattern of disease or phenotype, which has implications for diagnosis and a more personalized approach to therapy. Knowledge of the function and localization of the autoantigenic targets for MSAs has provided key insights into the potential immunopathogenic mechanisms of myositis. In particular, evidence suggests that the alteration of expression of a myositis-related autoantigen by certain environmental influences or oncogenesis could be a pivotal event linking autoantibody generation to the development of disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Larman, H. B. et al. Cytosolic 5′-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann. Neurol. 73, 408–418 (2013).
Pluk, H. et al. Autoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositis. Ann. Neurol. 73, 397–407 (2013).
Trallero-Araguas, E. et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 64, 523–532 (2012).
Mammen, A. L. et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 63, 713–721 (2011).
Betteridge, Z. & McHugh, N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J. Intern. Med. 280, 8–23 (2016).
Tansley, S. L. et al. Autoantibodies in juvenile-onset myositis: their diagnostic value and associated clinical phenotype in a large UK cohort. J. Autoimmun. 84, 55–64 (2017).
Love, L. A. et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine 70, 360–374 (1991).
Casciola-Rosen, L. et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J. Exp. Med. 201, 591–601 (2005).
Mohassel, P., Rosen, P., Casciola-Rosen, L., Pak, K. & Mammen, A. L. Expression of the dermatomyositis autoantigen transcription intermediary factor 1γ in regenerating muscle. Arthritis Rheumatol. 67, 266–272 (2015).
Amaral Silva, M., Cogollo, E. & Isenberg, D. A. Why do patients with myositis die? A retrospective analysis of a single-centre cohort. Clin. Exp. Rheumatol. 34, 820–826 (2016).
Johnson, C. et al. Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease. Lung 194, 733–737 (2016).
Koga, T. et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 51, 1278–1284 (2012).
Vancsa, A. et al. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol. Int. 29, 989–994 (2009).
La Corte, R., Lo Mo Naco, A., Locaputo, A., Dolzani, F. & Trotta, F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 39, 249–253 (2006).
Marie, I. et al. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum. 63, 3439–3447 (2011).
Witt, L. J., Curran, J. J. & Strek, M. E. The diagnosis and treatment of antisynthetase syndrome. Clin. Pulm. Med. 23, 218–226 (2016).
Nishikai, M. & Reichlin, M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 23, 881–888 (1980).
Mescam-Mancini, L. et al. Anti-Jo-1 antibody-positive patients show a characteristic necrotizing perifascicular myositis. Brain 138, 2485–2492 (2015).
Hamaguchi, Y. et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLOS ONE 8, e60442 (2013).
Klein, M. et al. Arthritis in idiopathic inflammatory myopathy: clinical features and autoantibody associations. J. Rheumatol. 41, 1133–1139 (2014).
Shi, J. et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J. Rheumatol. 44, 1051–1057 (2017).
Rider, L. G. et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine 92, 223–243 (2013).
Huber, A. M. et al. Early illness features associated with mortality in the juvenile idiopathic inflammatory myopathies. Arthritis Care Res. 66, 732–740 (2014).
Basharat, P. & Christopher-Stine, L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr. Rheumatol. Rep. 17, 72 (2015).
Pinal-Fernandez, I. et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res. 69, 263–270 (2017).
Kao, A. H., Lacomis, D., Lucas, M., Fertig, N. & Oddis, C. V. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 50, 209–215 (2004).
Hengstman, G. J. et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann. Rheum. Dis. 65, 1635–1638 (2006).
Suzuki, S. et al. Myopathy associated with antibodies to signal recognition particle: disease progression and neurological outcome. Arch. Neurol. 69, 728–732 (2012).
Binns, E. L. et al. Effective induction therapy for anti-SRP associated myositis in childhood: a small case series and review of the literature. Pediatr. Rheumatol. 15, 77 (2017).
Allenbach, Y. et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine 93, 150–157 (2014).
Mammen, A. L. Necrotizing myopathies: beyond statins. Curr. Opin. Rheumatol. 26, 679–683 (2014).
Tansley, S. L. et al. Anti-HMGCR autoantibodies in juvenile idiopathic inflammatory myopathies identify a rare but clinically important subset of patients. J. Rheumatol. 44, 488–492 (2017).
Kishi, T. et al. Association of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res. 69, 1088–1094 (2017).
Tiniakou, E. et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology 56, 787–794 (2017).
Sigurgeirsson, B., Lindelof, B., Edhag, O. & Allander, E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N. Engl. J. Med. 326, 363–367 (1992).
Hoshino, K. et al. Anti-MDA5 and anti-TIF1-γ antibodies have clinical significance for patients with dermatomyositis. Rheumatology 49, 1726–1733 (2010).
Fujimoto, M. et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 64, 513–522 (2012).
Ichimura, Y. et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann. Rheum. Dis. 71, 710–713 (2012).
Fiorentino, D. F. et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. 65, 2954–2962 (2013).
Albayda, J. et al. Dermatomyositis patients with anti-nuclear matrix protein-2 autoantibodies have more edema, more severe muscle disease, and increased malignancy risk. Arthritis Care Res., 69, 1771–1776 (2017).
Rogers, A., Chung, L., Li, S., Casciola-Rosen, L. & Fiorentino, D. F. Cutaneous and systemic findings associated with nuclear matrix protein 2 antibodies in adult dermatomyositis patients. Arthritis Care Res. 69, 1909–1914 (2017).
Kijanka, G. et al. Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut 59, 69–78 (2010).
Shah, A. A. et al. Evaluation of cancer-associated myositis and scleroderma autoantibodies in breast cancer patients without rheumatic disease. Clin. Exp. Rheumatol. 35 (Suppl. 106), 71–74 (2017).
Dutton, K. & Soden, M. Malignancy screening in autoimmune myositis amongst Australian rheumato-logists. Intern. Med. J. 47, 1367–1375 (2017).
Hengstman, G. J. et al. Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2β antigen. Ann. Rheum. Dis. 65, 242–245 (2006).
Ceribelli, A. et al. Myositis-specific autoantibodies and their association with malignancy in Italian patients with polymyositis and dermatomyositis. Clin. Rheumatol. 36, 469–475 (2017).
Allenbach, Y. et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain 139, 2131–2135 (2016).
Yang, H. et al. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res. Ther. 19, 259 (2017).
Watanabe, K. et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir. Med. 105, 1238–1247 (2011).
Aggarwal, R. et al. Myositis-associated usual interstitial pneumonia has a better survival than idiopathic pulmonary fibrosis. Rheumatology 56, 384–389 (2017).
Aggarwal, R. et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann. Rheum. Dis. 73, 227–232 (2014).
Hervier, B. et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 12, 210–217 (2012).
Lega, J. C. et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun. Rev. 13, 883–891 (2014).
Mahler, M. & Raijmakers, R. Novel aspects of autoantibodies to the PM/Scl complex: clinical, genetic and diagnostic insights. Autoimmun. Rev. 6, 432–437 (2007).
Sato, S. et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 60, 2193–2200 (2009).
Gono, T. et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology 51, 1563–1570 (2012).
Kobayashi, I. et al. Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J. Pediatr. 158, 675–677 (2011).
Kobayashi, N. et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology 54, 784–791 (2015).
Fiorentino, D., Chung, L., Zwerner, J., Rosen, A. & Casciola-Rosen, L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J. Am. Acad. Dermatol. 65, 25–34 (2011).
Hall, J. C. et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. 65, 1307–1315 (2013).
Moghadam-Kia, S., Oddis, C. V., Sato, S., Kuwana, M. & Aggarwal, R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res. 68, 689–694 (2016).
Tansley, S. L. et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res. Ther. 16, R138 (2014).
Narang, N. S., Casciola-Rosen, L., Li, S., Chung, L. & Fiorentino, D. F. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. 67, 667–672 (2015).
Fiorentino, D. F. et al. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J. Am. Acad. Dermatol. 72, 449–455 (2015).
Bailey, E. E. & Fiorentino, D. F. Amyopathic dermatomyositis: definitions, diagnosis, and management. Curr. Rheumatol. Rep. 16, 465 (2014).
Betteridge, Z. E. et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann. Rheum. Dis. 68, 1621–1625 (2009).
Sato, S. et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 52, 1571–1576 (2005).
Habers, G. E. et al. Association of myositis autoantibodies, clinical features, and environmental exposures at illness onset with disease course in juvenile myositis. Arthritis Rheumatol. 68, 761–768 (2016).
Tansley, S. L. et al. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology 53, 2204–2208 (2014).
Fredi, M. et al. Calcinosis in poly-dermatomyositis: clinical and laboratory predictors and treatment options. Clin. Exp. Rheumatol. 35, 303–308 (2017).
Ceribelli, A. et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res. Ther. 14, R97 (2012).
Valenzuela, A., Chung, L., Casciola-Rosen, L. & Fiorentino, D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 150, 724–729 (2014).
Benveniste, O. et al. Long-term observational study of sporadic inclusion body myositis. Brain 134, 3176–3184 (2011).
Lilleker, J. B. et al. Cytosolic 5′-nucleotidase 1A autoantibody profile and clinical characteristics in inclusion body myositis. Ann. Rheum. Dis. 76, 862–868 (2017).
Herbert, M. K. et al. Disease specificity of autoantibodies to cytosolic 5′-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann. Rheum. Dis. 75, 696–701 (2016).
Aggarwal, R. et al. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J. Rheumatol. 44, 223–229 (2016).
Bundell, C., Rojana-Udomsart, A., Mastaglia, F., Hollingsworth, P. & McLean-Tooke, A. Diagnostic performance of a commercial immunoblot assay for myositis antibody testing. Pathology 48, 363–366 (2016).
Cavazzana, I. et al. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods 433, 1–5 (2016).
O'Hanlon, T. P. et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1, and -DQA1 allelic profiles distinguish European American patients with different myositis autoantibodies. Medicine 85, 111–127 (2006).
Chinoy, H. et al. HLA-DPB1 associations differ between DRB1*03 positive anti-Jo-1 and anti-PM-Scl antibody positive idiopathic inflammatory myopathy. Rheumatology 48, 1213–1217 (2009).
Miller, F. W. et al. Genome-wide association study identifies HLA 8.1 ancestral haplotype alleles as major genetic risk factors for myositis phenotypes. Genes Immun. 16, 470–480 (2015).
Rothwell, S. et al. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann. Rheum. Dis. 75, 1558–1566 (2016).
Gono, T. et al. Association of HLA-DRB1*0101/*0405 with susceptibility to anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis in the Japanese population. Arthritis Rheum. 64, 3736–3740 (2012).
Targoff, I. N. et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 54, 3682–3689 (2006).
Mammen, A. L. et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 64, 1233–1237 (2012).
O'Hanlon, T. P. et al. HLA polymorphisms in African Americans with idiopathic inflammatory myopathy: allelic profiles distinguish patients with different clinical phenotypes and myositis autoantibodies. Arthritis Rheum. 54, 3670–3681 (2006).
Burd, C. J., Kinyamu, H. K., Miller, F. W. & Archer, T. K. UV radiation regulates Mi-2 through protein translation and stability. J. Biol. Chem. 283, 34976–34982 (2008).
Love, L. A. et al. Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis Rheum. 60, 2499–2504 (2009).
Okada, S. et al. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum. 48, 2285–2293 (2003).
Sarkar, K. et al. Seasonal influence on the onset of idiopathic inflammatory myopathies in serologically defined groups. Arthritis Rheum. 52, 2433–2438 (2005).
Morikawa, S. et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. J. Atheroscler. Thromb. 12, 121–131 (2005).
Watanabe, Y. et al. Statins and myotoxic effects associated with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies: an observational study in Japan. Medicine 94, e416 (2015).
Chinoy, H. et al. Interaction of HLA-DRB1*03 and smoking for the development of anti-Jo-1 antibodies in adult idiopathic inflammatory myopathies: a European-wide case study. Ann. Rheum. Dis. 71, 961–965 (2012).
Mammen, A. L. et al. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis Rheum. 60, 3784–3793 (2009).
Joseph, C. G. et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014).
Pinal-Fernandez, I. et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology 57, 388–396 (2018).
Aussy, A., Boyer, O. & Cordel, N. Dermatomyositis and immune-mediated necrotizing myopathies: a window on autoimmunity and cancer. Front. Immunol. 8, 992 (2017).
Pinal-Fernandez, I. et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology 56, 999–1007 (2017).
Aggarwal, R. et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatology 55, 991–999 (2016).
Vermaak, E., Tansley, S. L. & McHugh, N. J. The evidence for immunotherapy in dermatomyositis and polymyositis: a systematic review. Clin. Rheumatol. 34, 2089–2095 (2015).
Rider, L. G. & Miller, F. W. Laboratory evaluation of the inflammatory myopathies. Clin. Diagn. Lab. Immunol. 2, 1–9 (1995).
Stone, K. B. et al. Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum. 56, 3125–3131 (2007).
Muro, Y., Sugiura, K., Hoshino, K. & Akiyama, M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology 51, 800–804 (2012).
Mahler, M., Miller, F. W. & Fritzler, M. J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun. Rev. 13, 367–371 (2014).
Reichlin, M. & Mattioli, M. Description of a serological reaction characteristic of polymyositis. Clin. Immunol. Immunopathol. 5, 12–20 (1976).
Mathews, M. B., Reichlin, M., Hughes, G. R. & Bernstein, R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J. Exp. Med. 160, 420–434 (1984).
Reeves, W. H., Nigam, S. K. & Blobel, G. Human autoantibodies reactive with the signal-recognition particle. Proc. Natl Acad. Sci. USA 83, 9507–9511 (1986).
Bunn, C. C., Bernstein, R. M. & Mathews, M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J. Exp. Med. 163, 1281–1291 (1986).
Targoff, I. N., Trieu, E. P., Plotz, P. H. & Miller, F. W. Antibodies to glycyl-transfer RNA synthetase in patients with myositis and interstitial lung disease. Arthritis Rheum. 35, 821–830 (1992).
Targoff, I. N., Trieu, E. P. & Miller, F. W. Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J. Clin. Invest. 91, 2556–2564 (1993).
Hirakata, M. et al. Anti-KS: identification of autoantibodies to asparaginyl-transfer RNA synthetase associated with interstitial lung disease. J. immunol. 162, 2315–2320 (1999).
Hashish, L., Trieu, E. P., Sadanandan, P. & Targoff, I. N. Identification of autoantibodies to tyrosyl-tRNA synthetase in dermatomyositis with features consistent with antisynthetase syndrome. Arthritis Rheumatol. 52, S312 (2005).
Betteridge, Z., Gunawardena, H., North, J., Slinn, J. & McHugh, N. Anti-synthetase syndrome: a new autoantibody to phenylalanyl transfer RNA synthetase (anti-Zo) associated with polymyositis and interstitial pneumonia. Rheumatology 46, 1005–1008 (2007).
Betteridge, Z., Gunawardena, H., North, J., Slinn, J. & McHugh, N. Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum. 56, 3132–3137 (2007).
Targoff, I. N., Trieu, E. P., Levy-Neto, M. & Oddis, C. V. Sera with autoantibodies to MJ antigen react with NXP2. Arthritis Rheumatol. 56, S787 (2007).
Oddis, C. et al. Clinical and serological characterisation of the anti–MJ antibody in childhood myositis [abstract]. Arthritis Rheum. 40 (Suppl.), 139 (1997).
Chinoy, H. et al. In adult onset myositis, the presence of interstitial lung disease and myositis specific/associated antibodies are governed by HLA class II haplotype, rather than by myositis subtype. Arthritis Res. Ther. 8, R13 (2006).
Mierau, R. et al. Strong association of dermatomyositis-specific Mi-2 autoantibodies with a tryptophan at position 9 of the HLA-DR beta chain. Arthritis Rheum. 39, 868–876 (1996).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made a substantial contribution to discussions of content, wrote the article and contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
McHugh, N., Tansley, S. Autoantibodies in myositis. Nat Rev Rheumatol 14, 290–302 (2018). https://doi.org/10.1038/nrrheum.2018.56
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2018.56
This article is cited by
-
Anti-MDA5 antibody-positive dermatomyositis: pathogenesis and clinical progress
Nature Reviews Rheumatology (2024)
-
What should we expect when two myositis-specific antibodies coexist in a patient
European Journal of Medical Research (2023)
-
Pathogenesis of autoimmune disease
Nature Reviews Nephrology (2023)
-
Epidemiology of the idiopathic inflammatory myopathies
Nature Reviews Rheumatology (2023)
-
Two cases of dermatomyositis associated with neuroendocrine tumors
International Cancer Conference Journal (2023)