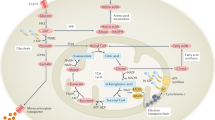
Key Points
-
Hypoxia, arising as a consequence of the increased cellular demand for oxygen during the inflammatory response, is a powerful trigger for the activation, proliferation and survival of endothelial cells and fibroblast-like synoviocytes
-
Impaired mitochondrial function and oxidative damage caused by hypoxia further exacerbate the inflammatory response through metabolic perturbation
-
Hypoxia induces immune cell dysfunction, resulting in an altered metabolic profile
-
The hypoxic environment induces activation of a complex crosstalk of signalling pathways, providing a feedback loop leading to further activation and inflammation
-
Targeting synovial metabolic pathways through inhibition of hypoxia-induced signalling pathways might have therapeutic benefit for rheumatoid arthritis and other inflammatory diseases
Abstract
Synovial proliferation, neovascularization and leukocyte extravasation transform the normally acellular synovium into an invasive tumour-like 'pannus'. The highly dysregulated architecture of the microvasculature creates a poor oxygen supply to the synovium, which, along with the increased metabolic turnover of the expanding synovial pannus, creates a hypoxic microenvironment. Abnormal cellular metabolism and mitochondrial dysfunction thus ensue and, in turn, through the increased production of reactive oxygen species, actively induce inflammation. When exposed to hypoxia in the inflamed joint, immune-inflammatory cells show adaptive survival reactions by activating key proinflammatory signalling pathways, including those mediated by hypoxia-inducible factor-1α (HIF-1α), nuclear factor κB (NF-κB), Janus kinase–signal transducer and activator of transcription (JAK–STAT) and Notch, which contribute to synovial invasiveness. The reprogramming of hypoxia-mediated pathways in synovial cells, such as fibroblasts, dendritic cells, macrophages and T cells, is implicated in the pathogenesis of rheumatoid arthritis and other inflammatory conditions, and might therefore provide an opportunity for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Semenza, G. L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 7, 345–350 (2001).
Prabhakar, N. R. Sensing hypoxia: physiology, genetics and epigenetics. J. Physiol. 591, 2245–2257 (2013).
Ng, C. T. et al. Synovial tissue hypoxia and inflammation in vivo. Ann. Rheum. Dis. 69, 1389–1395 (2010).
Kennedy, A. et al. Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum. 62, 711–721 (2010).
Kennedy, A. et al. Tumor necrosis factor blocking therapy alters joint inflammation and hypoxia. Arthritis Rheum. 63, 923–932 (2011).
Muller-Ladner, U., Gay, R. E. & Gay, S. Activation of synoviocytes. Curr. Opin. Rheumatol. 12, 186–194 (2000).
Koch, A. E. et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J. Immunol. 152, 4149–4156 (1994).
Asahara, T. et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ. Res. 83, 233–240 (1998).
Holash, J. et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998 (1999).
Malik, N. M. et al. Regulation of the angiopoietin- Tie ligand-receptor system with a novel splice variant of Tie1 reduces the severity of murine arthritis. Rheumatology (Oxford) 49, 1828–1839 (2010).
Chen, Y., Donnelly, E., Kobayashi, H., Debusk, L. M. & Lin, P. C. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 52, 1585–1594 (2005).
Saber, T. et al. Toll-like receptor 2 induced angiogenesis and invasion is mediated through the Tie2 signalling pathway in rheumatoid arthritis. PLoS ONE 6, e23540 (2011).
Szekanecz, Z., Besenyei, T., Szentpetery, A. & Koch, A. E. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr. Opin. Rheumatol. 22, 299–306 (2010).
Fearon, U. et al. Angiopoietins, growth factors, and vascular morphology in early arthritis. J. Rheumatol. 30, 260–268 (2003).
Biniecka, M. et al. Redox-mediated angiogenesis in the hypoxic joint of inflammatory arthritis. Arthritis Rheumatol. 66, 3300–3310 (2014).
Henrotin, Y. E., Bruckner, P. & Pujol, J. P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage 11, 747–755 (2003).
Ushio-Fukai, M. et al. Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 91, 1160–1167 (2002).
Vega, R. B., Horton, J. L. & Kelly, D. P. Maintaining ancient organelles: mitochondrial biogenesis and maturation. Circ. Res. 116, 1820–1834 (2015).
Stuart, J. A. & Brown, M. F. Mitochondrial DNA maintenance and bioenergetics. Biochim. Biophys. Acta 1757, 79–89 (2006).
Nakahira, K., Hisata, S. & Choi, A. M. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid. Redox Signal. 23, 1392–1350 (2015).
Filippin, L. I., Vercelino, R., Marroni, N. P. & Xavier, R. M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 152, 415–422 (2008).
Tsay, J. et al. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116, 2582–2589 (2010).
Bashir, S., Harris, G., Denman, M. A., Blake, D. R. & Winyard, P. G. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann. Rheum. Dis. 52, 659–666 (1993).
Lunec, J., Herbert, K., Blount, S., Griffiths, H. R. & Emery, P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 348, 131–138 (1994).
Hajizadeh, S., DeGroot, J., TeKoppele, J. M., Tarkowski, A. & Collins, L. V. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res. Ther. 5, R234–R240 (2003).
Biniecka, M. et al. Oxidative damage in synovial tissue is associated with in vivo hypoxic status in the arthritic joint. Ann. Rheum. Dis. 69, 1172–1178 (2010).
Vaamonde-Garcia, C. et al. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 64, 2927–2936 (2012).
Valcarcel-Ares, M. N. et al. Mitochondrial dysfunction promotes and aggravates the inflammatory response in normal human synoviocytes. Rheumatology (Oxford) 53, 1332–1343 (2014).
Onodera, Y., Teramura, T., Takehara, T., Shigi, K. & Fukuda, K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 5, 492–501 (2015).
Zhi, L., Ustyugova, I. V., Chen, X., Zhang, Q. & Wu, M. X. Enhanced TH17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J. Immunol. 189, 1639–1647 (2012).
Vermulst, M. et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 39, 540–543 (2007).
Biniecka, M. et al. Hypoxia induces mitochondrial mutagenesis and dysfunction in inflammatory arthritis. Arthritis Rheum. 63, 2172–2182 (2011).
Moodley, D., Mody, G., Patel, N. & Chuturgoon, A. A. Mitochondrial depolarisation and oxidative stress in rheumatoid arthritis patients. Clin. Biochem. 41, 1396–1401 (2008).
Da Sylva, T. R., Connor, A., Mburu, Y., Keystone, E. & Wu, G. E. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res Ther. 7, R844–R851 (2005).
Biniecka, M. et al. Successful tumour necrosis factor (TNF) blocking therapy suppresses oxidative stress and hypoxia-induced mitochondrial mutagenesis in inflammatory arthritis. Arthritis Res. Ther. 13, R121 (2011).
Bulua, A. C. et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208, 519–533 (2011).
Harty, L. C. et al. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 71, 582–588 (2012).
Deng, G. M., Nilsson, I. M., Verdrengh, M., Collins, L. V. & Tarkowski, A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5, 702–705 (1999).
Collins, L. V., Hajizadeh, S., Holme, E., Jonsson, I. M. & Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 75, 995–1000 (2004).
Zhang, J. Z., Liu, Z., Liu, J., Ren, J. X. & Sun, T. S. Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue. Int. J. Mol. Med. 33, 817–824 (2014).
Pazmandi, K. et al. Oxidative modification enhances the immunostimulatory effects of extracellular mitochondrial DNA on plasmacytoid dendritic cells. Free Radic. Biol. Med. 77, 281–290 (2014).
Li, Y. & Ye, D. Molecular biology for formyl peptide receptors in human diseases. J. Mol. Med. (Berl.) 91, 781–789 (2013).
Kao, W. et al. A formyl peptide receptor agonist suppresses inflammation and bone damage in arthritis. Br. J. Pharmacol. 171, 4087–4096 (2014).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Bours, M. J., Swennen, E. L., Di Virgilio, F., Cronstein, B. N. & Dagnelie, P. C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112, 358–404 (2006).
Miller, C. M. et al. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 7, e1002212 (2011).
Kato, M., Ospelt, C., Gay, R. E., Gay, S. & Klein, K. Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol. 66, 40–48 (2014).
Xu, K. et al. Autophagy induction contributes to the resistance to methotrexate treatment in rheumatoid arthritis fibroblast-like synovial cells through high mobility group box chromosomal protein 1. Arthritis Res. Ther. 17, 374 (2015).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Chang, X. & Wei, C. Glycolysis and rheumatoid arthritis. Int. J. Rheum. Dis. 14, 217–222 (2011).
Peansukmanee, S. et al. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J. Orthop. Res. 27, 529–535 (2009).
Ramakrishnan, P. et al. Oxidant conditioning protects cartilage from mechanically induced damage. J. Orthop. Res. 28, 914–920 (2010).
Henderson, B., Bitensky, L. & Chayen, J. Glycolytic activity in human synovial lining cells in rheumatoid arthritis. Ann. Rheum. Dis. 38, 63–67 (1979).
Naughton, D. et al. An investigation of the abnormal metabolic status of synovial fluid from patients with rheumatoid arthritis by high field proton nuclear magnetic resonance spectroscopy. FEBS Lett. 317, 135–138 (1993).
Ciurtin, C. et al. Correlation between different components of synovial fluid and pathogenesis of rheumatic diseases. Rom. J. Intern. Med. 44, 171–181 (2006).
Hitchon, C. A., El-Gabalawy, H. S. & Bezabeh, T. Characterization of synovial tissue from arthritis patients: a proton magnetic resonance spectroscopic investigation. Rheumatol. Int. 29, 1205–1211 (2009).
Firestein, G. S., Echeverri, F., Yeo, M., Zvaifler, N. J. & Green, D. R. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc. Natl Acad. Sci. USA 94, 10895–10900 (1997).
Tak, P. P., Zvaifler, N. J., Green, D. R. & Firestein, G. S. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol. Today 21, 78–82 (2000).
Tawakol, A. et al. HIF-1α and PFKFB3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler. Thromb. Vasc. Biol. 35, 1463–1471 (2015).
Ruscitti, P. et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3 (NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin. Exp. Immunol. 182, 35–44 (2015).
Makino, Y. et al. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J. Immunol. 171, 6534–6540 (2003).
Gaber, T. et al. Adaptation of human CD4+ T cells to pathophysiological hypoxia: a transcriptome analysis. J. Rheumatol. 36, 2655–2669 (2009).
Nakamura, H. et al. TCR engagement increases hypoxia-inducible factor-1α protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J. Immunol. 174, 7592–7599 (2005).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Dieckmann, D., Plottner, H., Berchtold, S., Berger, T. & Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193, 1303–1310 (2001).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Moran, E. M. et al. IL-17A expression is localised to both mononuclear and polymorphonuclear synovial cell infiltrates. PLoS ONE 6, e24048 (2011).
Dang, E. V. et al. Control of TH17/TREG balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and TREG cells. J. Exp. Med. 208, 1367–1376 (2011).
Basdeo, S. A. et al. Polyfunctional, pathogenic CD161+ TH17 lineage cells are resistant to regulatory T cell-mediated suppression in the context of autoimmunity. J. Immunol. 195, 528–540 (2015).
Clambey, E. T. et al. Hypoxia-inducible factor-1α-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl Acad. Sci. USA 109, E2784–E2793 (2012).
Shime, H. et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 180, 7175–7183 (2008).
Haas, R. et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13, e1002202 (2015).
Smith, M. D. et al. Standardisation of synovial tissue infiltrate analysis: how far have we come? How much further do we need to go? Ann. Rheum. Dis. 65, 93–100 (2006).
Cramer, T. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112, 645–657 (2003).
Fangradt, M. et al. Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia. Arthritis Res. Ther. 14, R181 (2012).
Burke, B. et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am. J. Pathol. 163, 1233–1243 (2003).
Lewis, J. S., Lee, J. A., Underwood, J. C., Harris, A. L. & Lewis, C. E. Macrophage responses to hypoxia: relevance to disease mechanisms. J. Leukoc. Biol. 66, 889–900 (1999).
Hamilton, J. A. et al. Hypoxia enhances the proliferative response of macrophages to CSF-1 and their pro-survival response to TNF. PLoS ONE 7, e45853 (2012).
Blagih, J. & Jones, R. G. Polarizing macrophages through reprogramming of glucose metabolism. Cell Metab. 15, 793–795 (2012).
Ricciardi, A. et al. Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol. Cancer Res. 6, 175–185 (2008).
Mancino, A. et al. Divergent effects of hypoxia on dendritic cell functions. Blood 112, 3723–3734 (2008).
Bosco, M. C. et al. Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood 117, 2625–2639 (2011).
Blengio, F. et al. The hypoxic environment reprograms the cytokine/chemokine expression profile of human mature dendritic cells. Immunobiology 218, 76–89 (2013).
Elia, A. R. et al. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J. Leukoc. Biol. 84, 1472–1482 (2008).
Yang, M. et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology 128, e237–e249 (2009).
Pierobon, D. et al. Chronic hypoxia reprograms human immature dendritic cells by inducing a proinflammatory phenotype and TREM-1 expression. Eur. J. Immunol. 43, 949–966 (2013).
Semenza, G. L. Life with oxygen. Science 318, 62–64 (2007).
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Brouwer, E. et al. Hypoxia inducible factor-1-alpha (HIF-1α) is related to both angiogenesis and inflammation in rheumatoid arthritis. Clin. Exp. Rheumatol. 27, 945–951 (2009).
Giatromanolaki, A. et al. Upregulated hypoxia inducible factor-1α and -2α pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 5, R193–R201 (2003).
Hu, F. et al. Hypoxia and hypoxia-inducible factor-1α provoke Toll-like receptor signalling-induced inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 73, 928–936 (2014).
Larsen, H., Muz, B., Khong, T. L., Feldmann, M. & Paleolog, E. M. Differential effects of TH1 versus TH2 cytokines in combination with hypoxia on HIFs and angiogenesis in RA. Arthritis Res. Ther. 14, R180 (2012).
Hot, A., Zrioual, S., Lenief, V. & Miossec, P. IL-17 and tumour necrosis factor α combination induces a HIF-1α-dependent invasive phenotype in synoviocytes. Ann. Rheum. Dis. 71, 1393–1401 (2012).
Li, G. et al. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α pathway. Mol. Immunol. 53, 227–236 (2013).
Lee, Y. A. et al. Hypoxia differentially affects IL-1β-stimulated MMP-1 and MMP-13 expression of fibroblast-like synoviocytes in an HIF-1α-dependent manner. Rheumatology (Oxford) 51, 443–450 (2012).
Jeon, C. H. et al. Hypoxia appears at pre-arthritic stage and shows co-localization with early synovial inflammation in collagen induced arthritis. Clin. Exp. Rheumatol. 26, 646–648 (2008).
del Rey, M. J. et al. Human inflammatory synovial fibroblasts induce enhanced myeloid cell recruitment and angiogenesis through a hypoxia-inducible transcription factor 1α/vascular endothelial growth factor-mediated pathway in immunodeficient mice. Arthritis Rheum. 60, 2926–2934 (2009).
Shi, M. et al. The protective effects of chronic intermittent hypobaric hypoxia pretreatment against collagen-induced arthritis in rats. J. Inflamm. (Lond.) 12, 23 (2015).
Darce, J. et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity 36, 731–741 (2012).
Wu, S. et al. Enhancement of angiogenesis through stabilization of hypoxia-inducible factor-1 by silencing prolyl hydroxylase domain-2 gene. Mol. Ther. 16, 1227–1234 (2008).
Muz, B., Larsen, H., Madden, L., Kiriakidis, S. & Paleolog, E. M. Prolyl hydroxylase domain enzyme 2 is the major player in regulating hypoxic responses in rheumatoid arthritis. Arthritis Rheum. 64, 2856–2867 (2012).
Liu, S. C. et al. CTGF increases vascular endothelial growth factor-dependent angiogenesis in human synovial fibroblasts by increasing miR-210 expression. Cell Death Dis. 5, e1485 (2014).
Gao, W. et al. Notch-1 mediates hypoxia-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 64, 2104–2113 (2012).
Coulon, C. et al. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler. Thromb. Vasc. Biol. 30, 2331–2336 (2010).
Thoms, B. L. & Murphy, C. L. Inhibition of hypoxia-inducible factor-targeting prolyl hydroxylase domain-containing protein 2 (PHD2) enhances matrix synthesis by human chondrocytes. J. Biol. Chem. 285, 20472–20480 (2010).
Tambuwala, M. M. et al. Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J. Control. Release 217, 221–227 (2015).
Zhou, J., Schmid, T. & Brune, B. Tumor necrosis factor-α causes accumulation of a ubiquitinated form of hypoxia inducible factor-1α through a nuclear factor-κB-dependent pathway. Mol. Biol. Cell 14, 2216–2225 (2003).
Jung, Y. J., Isaacs, J. S., Lee, S., Trepel, J. & Neckers, L. IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 17, 2115–2117 (2003).
Bruning, U. et al. NFκB and HIF display synergistic behaviour during hypoxic inflammation. Cell. Mol. Life Sci. 69, 1319–1329 (2012).
Oliver, K. M. et al. Hypoxia activates NF-κB-dependent gene expression through the canonical signaling pathway. Antioxid. Redox Signal. 11, 2057–2064 (2009).
Trebec-Reynolds, D. P., Voronov, I., Heersche, J. N. & Manolson, M. F. VEGF-A expression in osteoclasts is regulated by NF-κB induction of HIF-1α. J. Cell. Biochem. 110, 343–351 (2010).
Park, S. Y. et al. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. Eur. J. Immunol. 45, 1216–1227 (2015).
Cummins, E. P. et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl Acad. Sci. USA 103, 18154–18159 (2006).
Cockman, M. E. et al. Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH). Proc. Natl Acad. Sci. USA 103, 14767–14772 (2006).
Iso, T., Hamamori, Y. & Kedes, L. Notch signaling in vascular development. Arterioscler. Thromb. Vasc. Biol. 23, 543–553 (2003).
Hellstrom, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Diez, H. et al. Hypoxia-mediated activation of Dll4–Notch–Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp. Cell Res. 313, 1–9 (2007).
Gustafsson, M. V. et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 (2005).
Zheng, X. et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc. Natl Acad. Sci. USA 105, 3368–3373 (2008).
O'Shea, J. J. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity 7, 1–11 (1997).
Krause, A., Scaletta, N., Ji, J. D. & Ivashkiv, L. B. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J. Immunol. 169, 6610–6616 (2002).
Mori, T. et al. IL-1β and TNFα-initiated IL-6–STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int. Immunol. 23, 701–712 (2011).
Stump, K. L. et al. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res. Ther. 13, R68 (2011).
Gao, W. et al. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann. Rheum. Dis. 74, 1275–1283 (2015).
Oh, M. K. et al. Hypoxia-inducible factor-1α enhances haptoglobin gene expression by improving binding of STAT3 to the promoter. J. Biol. Chem. 286, 8857–8865 (2011).
Jung, J. E. et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 19, 1296–1298 (2005).
Jung, J. E. et al. STAT3 inhibits the degradation of HIF-1α by pVHL-mediated ubiquitination. Exp. Mol. Med. 40, 479–485 (2008).
Cai, Q., Verma, S. C., Kumar, P., Ma, M. & Robertson, E. S. Hypoxia inactivates the VHL tumor suppressor through PIASy-mediated SUMO modification. PLoS ONE 5, e9720 (2010).
Lee, J. H. et al. Notch signal activates hypoxia pathway through HES1-dependent SRC/signal transducers and activators of transcription 3 pathway. Mol. Cancer Res. 7, 1663–1671 (2009).
Qiang, L. et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 19, 284–294 (2012).
Morga, E. et al. Jagged1 regulates the activation of astrocytes via modulation of NFκB and JAK/STAT/SOCS pathways. Glia 57, 1741–1753 (2009).
Sone, H. et al. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem. Biophys. Res. Commun. 281, 562–568 (2001).
De Bandt, M. et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J. Immunol. 171, 4853–4859 (2003).
Wang, Y., Da, G., Li, H. & Zheng, Y. Avastin exhibits therapeutic effects on collagen-induced arthritis in rat model. Inflammation 36, 1460–1467 (2013).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Knowles, H. J., Tian, Y. M., Mole, D. R. & Harris, A. L. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1 α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 95, 162–169 (2004).
Semenza, G. L. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 33, 207–214 (2012).
Majumder, P. K. et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 10, 594–601 (2004).
Demaria, M. & Poli, V. PKM2, STAT3 and HIF-1α: the Warburg's vicious circle. JAKSTAT 1, 194–196 (2012).
Anastasiou, D. et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 8, 839–847 (2012).
Delgoffe, G. M. & Powell, J. D. mTOR: taking cues from the immune microenvironment. Immunology 127, 459–465 (2009).
Carames, B. et al. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 71, 575–581 (2012).
Zhang, Y. et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 74, 1432–1440 (2015).
Son, H. J. et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014, 973986 (2014).
Yan, H., Zhou, H. F., Hu, Y. & Pham, C. T. Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J. Rheum. Dis. Treat. 1, 5 (2015).
Treuhaft, P. S. & D. J., M. C. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 14, 475–484 (1971).
Lund-Olesen, K. Oxygen tension in synovial fluids. Arthritis Rheum. 13, 769–776 (1970).
Sivakumar, B. et al. Synovial hypoxia as a cause of tendon rupture in rheumatoid arthritis. J. Hand Surg. Am. 33, 49–58 (2008).
Author information
Authors and Affiliations
Contributions
All authors researched data for article and made substantial contribution to discussions of the content, writing and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fearon, U., Canavan, M., Biniecka, M. et al. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol 12, 385–397 (2016). https://doi.org/10.1038/nrrheum.2016.69
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.69
This article is cited by
-
Inhibiting HIF-1 signaling alleviates HTRA1-induced RPE senescence in retinal degeneration
Cell Communication and Signaling (2023)
-
Matrix metalloproteinases in arthritis: towards precision medicine
Nature Reviews Rheumatology (2023)
-
Metabolic reprogramming of proinflammatory macrophages by target delivered roburic acid effectively ameliorates rheumatoid arthritis symptoms
Signal Transduction and Targeted Therapy (2023)
-
Remodeling articular immune homeostasis with an efferocytosis-informed nanoimitator mitigates rheumatoid arthritis in mice
Nature Communications (2023)
-
SOX71, A Biocompatible Succinyl Derivative of the Triarylmethyl Radical OX071 for In Vivo Quantitative Oxygen Mapping Using Electron Paramagnetic Resonance
Molecular Imaging and Biology (2023)