Abstract
The intestinal immune system faces an extraordinary challenge from the large numbers of commensal bacteria and potential pathogens that are restrained by only a single layer of epithelial cells. Here, I discuss evidence that the intestinal immune system develops an extensive network of inducible, reversible lymphoid tissues that contributes to the vital equilibrium between the gut and the bacterial flora. I propose that this network is induced by cryptopatches, which are small clusters of dendritic cells and lymphoid cells that are identical to fetal inducers of lymph-node and Peyer's-patch development.
Similar content being viewed by others
Main
In the human intestine, there are 1014 bacteria, comprising more than 400 species, that feed on the food that we ingest and multiply1, yet this 'infection' is contained. In fact, we allow these bacteria to thrive and use their fermentation products as nutrients, as stimulators of intestinal absorption and as protective agents against pathogens and cancer1,2,3. Accordingly, the intestinal immune system is both vast and complex4. It has evolved not only to fight pathogens but also to confine the bacterial flora to the gut. To keep the digestive and absorptive functions of the gut at an optimum, a delicate equilibrium must be reached between the bacterial flora and the intestinal immune system2,3,5. At this equilibrium, lumenal bacteria do not cause considerable damage to, or invade, the single layer of gut epithelium, and the intestinal immune system does not destroy the bacterial population. However, this subtle harmony can be perturbed by changes in dietary habits, by ingestion of toxic compounds or by infection with pathogens, all of which can result in alterations to the composition of the gut flora, damage to the epithelium, infection of the intestinal tissues and/or induction of inflammation.
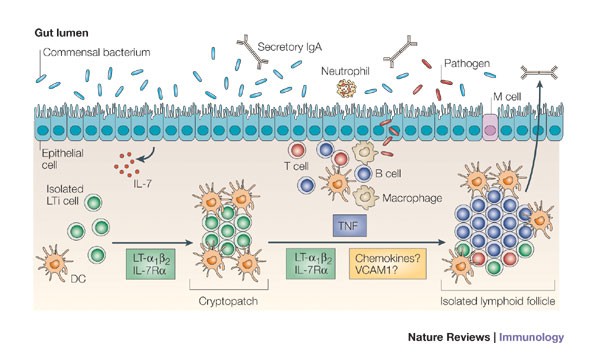
The intestinal immune system has developed a unique arsenal to maintain this equilibrium4,6 (Fig. 1). Many different cell types — including members of the innate and the adaptive immune systems, and specialized epithelial and mesenchymal cells — constantly interact to target bacteria in the gut lumen, epithelium or lamina propria. In particular, dendritic cells (DCs) sample antigen or live bacteria from these compartments and migrate to the T-cell zones of inductive sites, such as the mesenteric lymph nodes and the PEYER'S PATCHES7,8 (see Glossary). There, DCs can induce adaptive immune responses that lead to the generation of effector T cells and of plasma cells that produce IgA, the main immunoglobulin that is manufactured by the immune system and secreted in large amounts into the gut lumen.
A single layer of epithelial cells separates the intestinal lamina propria from the gut flora. It is protected by a thick layer of mucus, bactericidal defensins, neutrophils and large amounts of antigen-specific secretory IgA83. The intestinal immune system can be functionally divided into inductive sites, which include the mesenteric lymph nodes, Peyer's patches (in the small intestine), colonic patches17,89,90 and isolated lymphoid follicles (ILFs), and into effector sites, which include the epithelium and the lamina propria. In the epithelium, intraepithelial lymphocytes (IELs) monitor epithelial damage and might recognize microbial antigens26,27,91,92,93. The lamina propria contains large numbers of T cells, IgA-producing plasma cells and macrophages. It also contains many dendritic cells (DCs), which migrate to mesenteric lymph nodes through the lymph and present antigens to T cells4,6. The FOLLICLE-ASSOCIATED EPITHELIUM (FAE), which covers Peyer's patches, contains MICROFOLD (M) CELLS, which transport lumenal antigens to the sub-epithelial dome of Peyer's patches for sampling by DCs that can also sample antigens from apoptotic epithelial cells94,95. ILFs, similar to Peyer's patches, contain B cells, DCs and M cells (which are located in the adjacent FAE). Recent evidence indicates that cryptopatches, which contain DCs and Lin−cKIT+interleukin-7 receptor α-chain (IL-7Rα)+ cells, differentiate into ILFs12. Lin−cKIT+IL-7Rα+ cells are also found scattered in ILFs and in the sub-epithelial dome of Peyer's patches. HEV, high endothelial venule.
Recently, a new type of inductive site has been described, the isolated lymphoid follicle (ILF), which contains a single B-CELL FOLLICLE, as well as DCs and small numbers of T cells. ILFs are numerous in the lamina propria of the ANTI-MESENTERIC BORDER in mice, as well as in the most distal portion (the ileum) of the mouse small intestine9,10,11 and the colon12, and similar structures have been described for the human intestine13,14,15. In contrast to lymph nodes, Peyer's patches16 and COLONIC PATCHES17, ILFs do not develop during fetal life but form after birth, when the intestine is colonized by bacteria9,10.
It has been suggested that small clusters of lymphoid cells that reside between the CRYPTS of the mouse small intestine, known as cryptopatches, develop into ILFs when they are exposed to bacteria or pro-inflammatory stimuli12. Most cryptopatch cells have a phenotype and developmental requirements that are identical to those of fetal lymphoid-tissue inducer (LTi) cells12,18 (also known as LTICs19 or LOICs), which are essential for the initiation of lymph-node and Peyer's-patch development in the fetus16,20,21. Here, I propose that the young and adult intestinal immune system can recapitulate fetal lymphoid-tissue development and induce cryptopatches to form ILFs through the activation of LTi-like cells. The ability to form additional (and numerous) lymphoid follicles, and thereby markedly increase the number of lymphocytes that are present in the gut, might allow the intestine to adapt to a dynamic and potentially pathogenic gut flora and to maintain the crucial intestinal equilibrium.
Cryptopatches
Cryptopatches were first described following a study in mice by Ishikawa and colleagues, in 1996 (Ref. 18). They consist of small clusters of lymphoid cells (102–103 cells) that are located between the crypts in the lamina propria of the small intestine and, to a lesser extent, the colon, and they contain two main types of cell. Whereas 20–30% of cryptopatch cells are CD11c+ DCs, most cryptopatch cells resemble early T-cell precursors22, as they express cKIT, the interleukin-7 receptor α-chain (IL-7Rα), THY1 and CD44, but no T-cell receptor (TCR) or markers specific for the B-cell, macrophage, DC or granulocyte lineages12,18,23. Throughout this review, I will refer to these cells as lineage (Lin)−cKIT+IL-7Rα+ cryptopatch cells. In mice, cryptopatches appear 1–2 weeks after birth12,18, and ∼1,500 cryptopatches are found in the small intestine of adults18,23. They develop independently of gut flora, and they also develop independently of B and T cells, as they are present in mice that are deficient in recombination-activating gene 2 (RAG2), or the β- or δ-chain of the TCR, and in severe combined immunodeficient (SCID) mice18. Their development also does not require signalling through the nuclear factor-κB (NF-κB) pathway18, which is in contrast to the development of lymph nodes and Peyer's patches16. However, cryptopatches are absent in mice that are deficient in IL-7Rα18 or the common cytokine-receptor γ-chain, a component of the IL-7R23,24, indicating that IL-7 (which is produced by the intestinal epithelium25) has an essential role in the formation of cryptopatches.
The discovery of cryptopatches was akin to finding the holy grail for immunologists studying the intestinal immune system. For nearly two decades, the unique phenotype of CD8αα+ INTRAEPITHELIAL LYMPHOCYTES (IELs) (Fig. 1) and their presence in athymic mice, as well as the results of numerous graft experiments, indicated an extrathymic yet unidentified origin for these cells26,27,28. Several initial experiments indicated that cryptopatch cells were T-cell precursors and could differentiate into both αβ and γδ T cells23,29. Nevertheless, these conclusions have recently been challenged27, and genetic cell-fate mapping experiments show that cryptopatch cells are not T-cell precursors but, instead, might be adult counterparts of fetal LTi cells12.
Do cryptopatches generate T cells? An extrathymic origin for IELs was first inferred from their apparent lack of dependence on negative selection in the thymus30,31,32,33,34,35 and from their presence in athymic mice36. Furthermore, IELs could be derived from grafts of fetal liver or adult bone marrow given to athymic mice37,38,39. Bone-marrow grafts generated cryptopatches that first contained CD11c+ DCs, then Lin−cKIT+IL-7Rα+ cells, and this was followed by repopulation of the host with αβ-TCR+ and γδ-TCR+IELs23. In addition, the adoptive transfer of Lin−cKIT+IL-7Rα+ cryptopatch cells to SCID mice was shown to generate both αβ-TCR+ and γδ-TCR+ IELs, as well as T cells (but not B cells) in the lymph nodes, spleen and thymus29. As further evidence that cryptopatch cells were T-cell precursors, a small proportion (0.1%) of cryptopatch cells were shown to express transcripts encoding CD3ε, as well as the pre-TCR α-chain, RAG1 and RAG2 (Refs 24,40).
Several observations, however, favoured a thymic origin for IELs. The number of IELs, in particular the number of αβ-TCR+ IELs, is severely decreased in athymic mice, and athymic mice that receive thymic grafts generate IELs39,41,42,43,44. Moreover, recent experiments show that IELs can be positively selected in the thymus by agonist ligands, which negatively select for mature T cells in the thymus, spleen and lymph nodes45. Similarly, re-aggregated thymic cultures generate IEL-like CD8αα+ cells in the presence of agonist ligands46. Further compelling evidence for a thymic origin of αβ-TCR+ IELs in normal mice was obtained when the progeny of both CD4+CD8+ double positive (DP) immature thymocytes and Lin−cKIT+IL-7Rα+ cryptopatch cells were determined by genetic cell-fate mapping12. It was found that both DP thymocytes and Lin−cKIT+IL-7Rα+ cryptopatch cells are unique in expressing the nuclear hormone receptor ROR-γt (retinoic-acid-receptor-related orphan receptor-γt), whereas DP thymocytes, but not Lin−cKIT+IL-7Rα+ cryptopatch cells, were found to be able to express a transgene under the control of a recombinant Cd4 promoter. Mice were then generated that expressed the Cre recombinase under the control of the Rorc locus (which encodes ROR-γt) or the recombinant Cd4 promoter, and these were crossed with a reporter mouse line that expressed green fluorescent protein only in cells harbouring Cre and their progeny47. It was concluded from these experiments that all αβ-TCR+ IELs, but not γδ-TCR+ IELs, are derived from DP thymocytes and that cryptopatch cells generate neither αβ T cells nor γδ T cells. Nevertheless, it remains possible that a subset of Lin−cKIT+IL-7Rα+ cryptopatch cells generate αβ and γδ T cells on transfer to SCID mice, owing to the lymphopaenic state of these recipients. Accordingly, although extrathymic generation of IELs seems to be minimal in euthymic mice, in the absence of a thymus, this pathway is considerably more productive, in particular in the mesenteric lymph nodes48. Finally, subsets of γδ-TCR+ IELs are likely to derive from CD3−CD4−CD8− triple-negative thymocytes27,49, but an intestinal origin for γδ-TCR+ IELs has not been ruled out by these studies. Interestingly, it has recently been shown that (αβ-TCR+) DP thymocytes regulate the differentiation of γδ T cells in the thymus by a lymphotoxin-β receptor (LT-βR)-dependent mechanism, and it was suggested that cryptopatch cells might similarly regulate the differentiation of intestinal γδ T cells50.
Do cryptopatches contain LTi-like cells? If cryptopatches do not contain T-cell precursors, then what are Lin−cKIT+IL-7Rα+ cryptopatch cells? These cells express the nuclear hormone receptor ROR-γt12, as do DP thymocytes in the adult and LTi cells in the fetus20. LTi cells are among the first haematopoietic cells to colonize developing lymph nodes and Peyer's patches (in regions known as the lymph-node and Peyer's-patch anlagen)20,51,52; this occurs 1 week before birth and days before lymphocytes are recruited and segregate into B- and T-cell zones. LTi cells also express the membrane-bound heterotrimer lymphotoxin-α1β2 (LT-α1β2)51,52, which is essential for the activation of local mesenchymal cells through the LT-βR53,54,55,56. Activated mesenchymal cells are then involved in the formation of mature lymphoid tissues by recruiting (presumably through chemokines and adhesion molecules) new waves of LTi cells and, at a later stage of ontogeny, B cells, T cells and DCs16 (Fig. 2). Remarkably, fetal LTi cells, clustered in the lymph-node and Peyer's-patch anlagen, and adult intestinal Lin−cKIT+IL-7Rα+ cells, clustered in cryptopatches, express an identical set of markers12. In addition, the generation of both cell types depends on expression of ROR-γt and the inhibitor of basic helix-loop-helix transcription factors ID2 (inhibitor of DNA binding 2), but it is independent of RAG proteins and LT-α. There is also data that indicates a direct lineage relationship between fetal LTi cells and intestinal Lin−ckit+IL-7Rα+ cryptopatch cells. Although LTi cells are abundant in lymph-node and Peyer's-patch anlagen before birth, there are few of these cells present within days of birth, and they cannot be detected in the lymph nodes and Peyer's patches of adults20,51. However, isolated LTi cells are found in the intestinal lamina propria after birth, and they cluster into cryptopatches at 1–2 weeks of age12 (Fig. 3). Therefore, it has been proposed that Lin−ckit+IL-7Rα+ cryptopatch cells are the adult counterpart of fetal LTi cells12.
a | Lymphoid-tissue inducer (LTi) cells are required for initiation of lymph-node and Peyer's-patch development in the fetus. They were originally described as CD3−CD4+CD45+ cells96, and they are derived from fetal liver-cell precursors97,98. These cells are recruited to the lymph-node and Peyer's-patch anlagen, probably by chemokines — such as CXC-chemokine ligand 13 (CXCL13), which interacts with CXC-chemokine receptor 5 (CXCR5) — and they interact with specialized mesenchymal cells, through cell-surface-expressed α4-integrins16. LTi cells express lymphotoxin-α1β2 (LT-α1β2), the expression of which is upregulated by interleukin-7 (IL-7) or other ligands of the IL-7 receptor α-chain (IL-7Rα)64, and they activate mesenchymal cells through the LT-β receptor (LT-βR). As a result, mesenchymal cells produce more chemokines (including CXCL13, CC-chemokine ligand 19 (CCL19) and CCL21), express intercellular adhesion molecule 1 (ICAM1) and vascular cell-adhesion molecule 1 (VCAM1), and establish the first positive-feedback loop for the recruitment of LTi cells. A few days before birth, lymphocytes and dendritic cells (DCs) are recruited to the lymph-node and Peyer's-patch anlagen. Because T cells and B cells themselves express LT-α1β2 (Ref. 80), recruitment of lymphocytes is amplified by a second positive-feedback loop and culminates in the formation of a mature lymph node or Peyer's patch, with segregated T-cell zones and B-cell follicles, high endothelial venules (HEVs) and additional specialized mesenchymal cells. b | Evidence indicates that the formation of isolated lymphoid follicles in the young and adult intestine recapitulates the fetal development of lymph nodes and Peyer's patches12.
After birth, lymphoid-tissue inducer (LTi)-like Lin−ckit+interleukin-7 receptor α-chain (IL-7Rα)+ cells are present in the lamina propria of the small intestine and the colon, and they express the nuclear receptor ROR-γt (retinoic-acid-receptor-related orphan receptor-γt)12. Frozen sections were prepared from the intestines of mice expressing the enhanced green fluorescent protein under control of the ROR-γt promoter; therefore, Lin−ckit+IL-7Rα+ cells expressing ROR-γt were visible under fluorescent light (green). These sections were then stained to visualize CD11c, present at the cell surface of dendritic cells (DCs) (red), and B220, present at the cell surface of B cells (blue). One week after birth, ROR-γt+ cells are scattered throughout the lamina propria, but during the second week, they cluster into cryptopatches, together with DCs. At the beginning of the third week, cryptopatches in the colon, but not in the small intestine, seem to differentiate into small isolated lymphoid follicles (ILFs) as they recruit B cells, and they develop further into large ILFs at the end of the third week. The formation of ILFs depends on the presence of gut flora, tumour-necrosis-factor receptor 1, IL-7Rα and lymphotoxin-α1β2. The generation of Lin−ckit+IL-7Rα+ cells, as well as the formation of cryptopatches and ILFs, depends on ROR-γt.
Organizers of lymphoid tissue
I propose a novel hypothesis for the function of cryptopatches. Because cryptopatches do not generate T cells but, instead, might contain LTi-like cells, I suggest that cryptopatches are adult organizers of intestinal lymphoid tissue. Lin−ckit+IL-7Rα+ cryptopatch cells might induce the formation of ILFs and might recapitulate the fetal induction of lymph-node and Peyer's-patch development by LTi cells16,21 (Fig. 2).
Several lines of evidence support this hypothesis. First, as mentioned earlier, intestinal Lin−ckit+IL-7Rα+ cells cluster into cryptopatches during the second week after birth12. At the beginning of the third week, the number of cryptopatches in the colon decreases (Fig. 3), and small ILFs appear, which develop into large ILFs at the end of the third week, shortly before weaning9,10 (Fig. 3). Second, in ROR-γt-deficient mice, the lack of lymph nodes and Peyer's patches is a consequence of the absence of fetal LTi cells20. ROR-γt-deficient mice also fail to generate adult intestinal Lin−ckit+IL-7Rα+ cells, as well as cryptopatches and ILFs12. Third, lymph-node and Peyer's-patch development requires the expression of LT-α1β2 by LTi cells20,52,57. By contrast, the formation of ILFs is independent of LT-α1β2 expressed by fetal LTi cells but requires LT-α1β2 after birth9,10,58. Together, these data indicate that Lin−ckit+IL-7Rα+ cells in cryptopatches are required for the formation of ILFs by an LT-α1β2-dependent mechanism.
Additional factors that are involved in the induction of lymph-node and Peyer's-patch development by LTi cells also seem to be involved in ILF formation. For example, IL-7Rα is required for the development of lymph nodes and Peyer's patches59,60, as well as for the formation of ILFs9. Both LTi cells and Lin−ckit+IL-7Rα+ cryptopatch cells express IL-7Rα18,51,61, and signalling through this receptor has been shown to induce upregulation of LT-α1β2 expression57,60,62. In the fetus, IL-7 is expressed in the thymus, in the liver, in the intestine63 and by the specialized mesenchymal cells of the Peyer's-patch anlagen64. In the adult gut, IL-7 is produced by the epithelium25,65. Nevertheless, because the development of Peyer's patches is initiated in IL-7-deficient mice but not in IL-7Rα-deficient mice, it has been proposed that additional ligands of IL-7Rα are involved in lymphoid-tissue development64. Furthermore, fetal LTi cells activate the mesenchymal cells of lymph-node and Peyer's-patch anlagen to express intercellular adhesion molecule 1 (ICAM1) and vascular cell-adhesion molecule 1 (VCAM1)16,20. Whereas cryptopatches do not express (or express only low levels of) ICAM1 and VCAM1, small immature and large mature ILFs express high levels of these adhesion molecules12,58, implying that the mesenchymal cells in these ILFs are activated. Finally, tumour-necrosis factor (TNF) receptor 1 (TNFR1), which is involved in the development of Peyer's patches16, is also required for the formation of ILFs10. As well as TNF, other TNF-superfamily members — including LT-α3, TRANCE (TNF-related activation-induced cytokine) and LIGHT — and CXC-chemokine ligand 13 (CXCL13; also known as BLC) have also been shown to be involved in lymph-node and Peyer's-patch development16. It will be important to assess whether these factors are also involved in the formation of ILFs and whether the proposed differentiation of cryptopatches into ILFs recapitulates all of the aspects of lymph-node and Peyer's-patch development (Fig. 4).
Fetal intestinal lymphoid-tissue inducer (LTi) cells might persist in the lamina propria of newborns, where they could cluster into cryptopatches, together with dendritic cells (DCs), during the second week of life. The formation of cryptopatches depends on the interleukin-7 receptor α-chain (IL-7Rα) and on lymphotoxin-α1β2 (LT-α1β2), which is possibly expressed by LTi-like cells, but it is independent of the gut flora12,18,58. In response to a growing local gut flora, colonic cryptopatches differentiate into isolated lymphoid follicles (ILFs) during the second to third week of life9,10 (Fig. 3). The formation of ILFs requires LTi-like cells, LT-α1β2 and IL-7Rα, as well as the gut flora and tumour-necrosis factor (TNF)9,10,12. It remains to be determined whether chemokines and adhesion molecules, such as intercellular adhesion molecule 1 (ICAM1) and vascular cell-adhesion molecule 1 (VCAM1), expressed by mesenchymal cells are required for ILF formation, similar to lymph-node and Peyer's-patch development (Fig. 2). Increased numbers of ILFs, which are also hyperplastic, form in the small intestine in response to excessive numbers of gut flora68 or during inflammation14,66,67,79, both of which are perturbations that could result in the production of TNF. Pathogens, such as Salmonella spp. and Yersinia spp., are taken up by DCs present in ILFs and can elicit antigen-specific IgA responses15. ILFs might also produce IgA to maintain homeostasis of the gut flora, as well as in response to inflammation. M cell, microfold cell.
Cryptopatches as inducible organizers
Despite these similarities, there is a fundamental difference between the formation of ILFs after birth and the fetal development of lymph nodes and Peyer's patches. The development of lymph nodes and Peyer's patches is initiated at precise time points and locations during fetal ontogeny16, independently of microbial stimulation. By contrast, the formation of ILFs is induced by intestinal bacteria9,10 or inflammation14,66,67 (Fig. 4). That is, lymph nodes and Peyer's patches are programmed secondary lymphoid tissues, whereas ILFs are inducible tertiary lymphoid tissues. Primary lymphoid tissues are sites of lymphocyte generation, such as the liver in the fetus and the bone marrow and thymus in the adult. Therefore, although Lin−ckit+ IL-7Rα+ cryptopatch cells might be functional equivalents of LTi cells, unlike fetal LTi cells, they seem to require activation to induce the formation of ILFs. For example, because the development of an intestinal flora is required for the formation of mature ILFs in young mice9,10, it is possible that bacteria induce the expression of cytokines by the epithelium, by macrophages or by DCs present in the epithelium, lamina propria or cryptopatches. These cytokines could then activate the LTi-like function of Lin−ckit+IL-7Rα+ cryptopatch cells.
The formation of ILFs in the colon after birth favours the hypothesis that cryptopatches are inducible organizers of ILFs. Cryptopatches are abundant in the small intestine of adult mice but are rare in the colon18. Conversely, ILFs are abundant in the colon (Ref. 12, and G.E., unpublished observations) but are found only in particular regions of the small intestine, such as the anti-mesenteric wall and the ileum9,10,11. As discussed earlier, histological analysis indicates that, during the third week of life, colonic cryptopatches differentiate into ILFs (Fig. 3). Because the number of gut bacteria increases during the first weeks of life and is highest in the ileum and the colon, these observations indicate that ILFs develop from cryptopatches in response to an increased load of lumenal bacteria. Furthermore, an abnormally high load of lumenal bacteria can lead to ILF hyperplasia. Mice that are deficient in activation-induced cytidine deaminase (AID), which is required for somatic hypermutation and class-switch recombination of immunoglobulin genes, fail to produce IgA and affinity-matured IgM68. As a consequence, these mice have 100-fold more bacteria present in the upper parts of the small intestine (the duodenum and jejunum), as well as increased numbers of ILFs that are also markedly hyperplastic. A similar phenotype is found in patients with a deficiency in AID69. Conversely, germ-free mice have only immature ILFs that mainly contain Lin−cKIT+IL-7Rα+ cells9 and are structurally similar to cryptopatches.
The formation of ILFs not only is inducible but also is reversible. Adult mice treated with an LT-βR–immunoglobulin fusion protein (a decoy receptor for LT-α1β2) have no ILFs in the small intestine, and mice treated with a TNFR1–immunoglobulin fusion protein (a decoy receptor for TNF and LT-α3) have a 2–3-fold decrease in the number of ILFs that are present70. Furthermore, treatment of AID-deficient mice with antibiotics to reduce the intestinal bacterial load abolishes ILF hyperplasia and reduces the number of ILFs68. These observations show that signalling through TNFR1, as well as signals that are mediated by bacteria and LT-α1β2, not only are required for the formation of ILFs but also are essential for their maintenance.
Mechanisms for the formation of ILFs
The gut flora induces ILF formation, and TNFR1 ligands (such as TNF and LT-α3) are also required for this process9,10. These observations could indicate that the formation of ILFs involves Toll-like receptors (TLRs), which bind microbial components and induce expression of pro-inflammatory factors, such as TNF, type I interferons, T-helper-1-cell cytokines and prostaglandins71. TLRs are expressed by many cell types, including intestinal epithelial cells, DCs and macrophages. Intestinal epithelial cells express varied levels of TLR2 (a receptor for lipoproteins and glycolipids), TLR3 (a receptor for double-stranded RNA), TLR4 (a receptor for lipopolysaccharide and heat-shock proteins) and TLR5 (a receptor for flagellin)71,72,73,74. DCs express different types of TLR depending on the subset and their state of maturity, whereas macrophages express most TLRs71. Given this, DCs that are present in cryptopatches (constituting 20–30% of cryptopatch cells) might bind bacterial products or components through TLRs, after the penetration of these molecules or live bacteria into the lamina propria. Additional mechanisms could be involved in the detection of intestinal bacteria and the production of pro-inflammatory signals, such as the uptake of live bacteria7,8 or bacteria opsonized with IgA75 by neutrophils, myeloid cells and DCs.
So, in the model proposed here, how would cryptopatches integrate pro-inflammatory signals and induce the formation of ILFs? Cryptopatches are organized as a central cluster of Lin−ckit+IL-7Rα+ cells (which constitutes 70–80% of cryptopatch cells) and a ring of DCs18. TNF that is produced by various cells that have been activated through TLRs, or by DCs in cryptopatches, might directly activate Lin−ckit+IL-7Rα+ cryptopatch cells. In an indirect manner, TNF might also induce the maturation of DCs76 in cryptopatches, which would then activate Lin−ckit+IL-7Rα+ cryptopatch cells. As a consequence, activated Lin−ckit+IL-7Rα+ cryptopatch cells might upregulate expression of LT-α1β2, and similar to the function of LTi cells in the development of lymph nodes and Peyer's patches16 (Fig. 2), this could then induce LT-βR-expressing cryptopatch mesenchymal cells to secrete lymphocyte-recruiting chemokines — such as CXCL13, CC-chemokine ligand 19 (CCL19; also known as ELC) and CCL21 (also known as SLC) — and to express high levels of ICAM1 and VCAM1. Similar to lymph-node and Peyer's-patch development, this would culminate in the formation of an organized lymphoid tissue (Fig. 4).
Not only can both intestinal flora and pathogens induce the production of TNF, but high levels of TNF are also produced during inflammatory bowel disease (IBD). This cytokine is an important factor in IBD, because administration of TNF-specific antibody is now a standard treatment for IBD77. In chemically66 or genetically78 induced colitis in mice, ILFs become enlarged and are increased in number. Moreover, in ulcerative colitis and Crohn's disease in humans, hyperplastic lymphoid aggregates have been observed in the colonic lamina propria, and these mainly contain B cells, T cells and DCs14,67,79. These structures are similar to ILFs, and they indicate that the large amount of TNF produced during IBD might induce the hyperplasia of ILFs. It should be noted that TNF and other pro-inflammatory factors (such as LT-α1β2 and LT-α3) can be expressed by various cells, including activated T and B cells that are recruited to the site of chronic inflammation80,81. So, it is possible that these activated cells induce ILF formation independently of Lin−cKIT+IL-7Rα+ cryptopatch cells.
Role of cryptopatches and ILFs
From the structural similarity between ILFs and B-cell follicles in lymph nodes and Peyer's patches, and from the location of ILFs beneath the intestinal epithelium9,10,12, it can be inferred that ILFs have a role in the intestinal immune system that is similar to that of Peyer's patches: that is, they are immune inductive sites. Indeed, it has recently been reported that ILFs can induce the production of antigen-specific IgA15,70. However, in contrast to Peyer's patches, ILFs are inducible structures9,10,68. Their size varies from clusters that are the size of cryptopatches (102–103 cells), in the lower part of the normal small intestine, to clusters that are approximately one-quarter the size of normal Peyer's-patch follicles, in Peyer's-patch-deficient mice10 and in the colon12. Most markedly, in AID-deficient mice, ILFs in the upper small intestine reach the size of normal Peyer's-patch follicles (105–106 cells)68. On average, a mouse small intestine has 8–12 Peyer's patches, each of which contains 5–7 B-cell follicles; therefore, there are a total of 40–84 follicles, which contain a total of 0.5 × 107–108 cells, in the small intestine. By contrast, an estimated 150–300 ILFs are present in the small intestine9,10, which contain a total of ∼106 cells in normal mice and almost 108 cells in AID-deficient mice68, thereby possibly outnumbering the cells present in the Peyer's patches. Therefore, in response to a new threat, the inducible nature of ILFs allows the mobilization of a large number of lymphocytes (mainly B cells9) in addition to those that are already present in the Peyer's patches and mesenteric lymph nodes.
ILFs are inducible9,10,68 and reversible lymphoid tissues68,70, which can generate IgA-mediated antigen-specific responses15,70. Because these responses quench their own source of induction, ILFs might self-regulate their number and size. For example, an increase in the number of bacteria present in the lumen induces an increase in the number and size of ILFs, which in turn generates an IgA-mediated response that targets the bacteria. However, as bacteria are neutralized, the induction of ILFs decreases, leading to an equilibrium between bacteria and ILFs. In AID-deficient mice, the number of lumenal bacteria present in the small intestine is 100-fold greater than in normal mice; in response, the number and size of ILFs are also markedly increased68. Again, both parameters stabilize and reach an equilibrium; however, this is different from the equilibrium in normal mice. Nevertheless, AID-deficient mice have seemingly normal intestinal functions68,82 and have no intestinal inflammation, showing the exceptional flexibility of intestinal structure and function. Cryptopatches and ILFs might therefore have an essential role in the equilibrium that is reached between the gut flora and the immune system.
The specific role of cryptopatches and ILFs in immunity to intestinal pathogens and in establishing tolerance is starting to be addressed. Lorenz and Newberry15 have recently shown that ILFs can induce the production of Salmonella typhimurium-specific IgA after oral infection with these bacteria. They also showed that Yersinia enterocolitica is found in mature ILFs 24 hours after oral infection. It will be important to assess further the role of cryptopatches and ILFs during infection with intestinal pathogens (such as Shigella spp., Toxoplasma gondii and reoviruses1,83) and to determine the involvement of cryptopatches and ILFs in the establishment of tolerance to orally administered antigens84,85.
By contrast, cryptopatches and ILFs might also be involved in intestinal immunopathology. As ILF hyperplasia is induced during IBD14,66,67,79, it is possible that cryptopatches and ILFs exacerbate IBD pathology by establishing a positive-feedback loop for inflammation. In this case, if the differentiation of cryptopatches into ILFs could be inhibited, this could lead to a novel class of treatments for IBD. For example, because ROR-γt is specifically expressed by intestinal Lin−cKIT+IL-7Rα+ cells (and by immature thymocytes)12 and because it is required for the formation of cryptopatches and ILFs, it has been proposed that ROR-γt could be a pharmacological target for the treatment of IBD12.
Conclusions
In this Opinion article, I have developed the idea that cryptopatches are intestinal 'sensors' of the gut flora, as well as of intestinal infection and injury. Pro-inflammatory signals might activate LTi-like cryptopatch cells to induce the formation of ILFs, recapitulating the fetal pathway of lymph-node and Peyer's-patch development. The intestinal cryptopatch–ILF pathway could thereby ensure the formation of the number of inductive lymphoid tissues that is appropriate to the number of lumenal bacteria or to the challenge posed by infection or injury. In addition, cryptopatches might regulate the differentiation of intestinal T cells24,40,50.
Tertiary lymphoid tissues also form in chronic autoimmune lesions86,87 and in the lungs after infection with influenza virus88. In the latter case, however, the formation of inducible bronchus-associated lymphoid tissues is independent of ROR-γt, so it presumably does not require LTi-like cells. It is possible that, during infection and chronic inflammation, the recruitment of high numbers of activated T and B cells expressing LT-α1β2, TNF80 or other pro-inflammatory factors bypasses the requirement for LTi-like cells in the formation of tertiary lymphoid tissue.
So, the intestine might have maintained the unique fetal characteristics of lymphoid-tissue development. Nevertheless, it is an optimal and physiological model to study the formation and the function of tertiary, inducible lymphoid tissues. Evolution of the intestinal cryptopatch–ILF network can be readily monitored and studied in response to bacterial colonization of the gut and perturbations in the gut flora, as well as in response to well-characterized pathogens and tumours, and during chronic inflammatory disease. Eventually, the cryptopatch–ILF model could generate knowledge that leads to the design of new therapies that increase specific immunity through the local formation of tertiary lymphoid tissues or that prevent chronic inflammation in IBD and autoimmunity.
References
Ogra, P. L. et al. (eds) Mucosal Immunology (Academic, San Diego, 1999).
Guarner, F. & Malagelada, J. R. Gut flora in health and disease. Lancet 361, 512–519 (2003).
Hooper, L. V. & Gordon, J. I. Commensal host–bacterial relationships in the gut. Science 292, 1115–1118 (2001).
Mowat, A. M. Anatomical basis of tolerance and immunity to intestinal antigens. Nature Rev. Immunol. 3, 331–341 (2003).
McCracken, V. J. & Lorenz, R. G. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 3, 1–11 (2001).
Brandtzaeg, P. & Pabst, R. Let's go mucosal: communication on slippery ground. Trends Immunol. 25, 570–577 (2004).
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
Lorenz, R. G., Chaplin, D. D., McDonald, K. G., McDonough, J. S. & Newberry, R. D. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J. Immunol. 170, 5475–5482 (2003).
Pabst, O. et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 35, 98–107 (2005).
Eberl, G. & Littman, D. R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Moghaddami, M., Cummins, A. & Mayrhofer, G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 115, 1414–1425 (1998).
Yeung, M. M. et al. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TCR-γδ expression. Gut 47, 215–227 (2000).
Lorenz, R. G. & Newberry, R. D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. NY Acad. Sci. 1029, 44–57 (2004).
Mebius, R. E. Organogenesis of lymphoid tissues. Nature Rev. Immunol. 3, 292–303 (2003).
Dohi, T. et al. Elimination of colonic patches with lymphotoxin β receptor–Ig prevents TH2 cell-type colitis. J. Immunol. 167, 2781–2790 (2001).
Kanamori, Y. et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 184, 1449–1459 (1996).
Cyster, J. G. Lymphoid organ development and cell migration. Immunol. Rev. 195, 5–14 (2003).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nature Immunol. 5, 64–73 (2004).
Eberl, G. & Littman, D. R. The role of the nuclear hormone receptor RORγt in the development of lymph nodes and Peyer's patches. Immunol. Rev. 195, 81–90 (2003).
Kondo, M. et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 (2003).
Suzuki, K. et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 13, 691–702 (2000).
Oida, T. et al. Role of gut cryptopatches in early extrathymic maturation of intestinal intraepithelial T cells. J. Immunol. 164, 3616–3626 (2000).
Watanabe, M. et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95, 2945–2953 (1995).
Guy-Grand, D. & Vassalli, P. Gut intraepithelial T lymphocytes. Curr. Opin. Immunol. 5, 247–252 (1993).
Guy-Grand, D. & Vassalli, P. Gut intraepithelial lymphocyte development. Curr. Opin. Immunol. 14, 255–259 (2002).
Rocha, B., Guy-Grand, D. & Vassalli, P. Extrathymic T cell differentiation. Curr. Opin. Immunol. 7, 235–242 (1995).
Saito, H. et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 280, 275–278 (1998).
Rocha, B., Vassalli, P. & Guy-Grand, D. The Vβ repertoire of mouse gut homodimeric αα CD8+ intraepithelial T cell receptor αβ+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J. Exp. Med. 173, 483–486 (1991).
Rocha, B., von Boehmer, H. & Guy-Grand, D. Selection of intraepithelial lymphocytes with CD8αα co-receptors by self-antigen in the murine gut. Proc. Natl Acad. Sci. USA 89, 5336–5340 (1992).
Lin, T. et al. Autospecific γδ thymocytes that escape negative selection find sanctuary in the intestine. J. Clin. Invest. 104, 1297–1305 (1999).
Badiner, G., Goodman, T. G. & Lefrancois, L. Selection of intestinal intraepithelial lymphocyte T cell receptors: evidence for a dynamic tissue-specific process. Int. Immunol. 5, 223–226 (1993).
Cruz, D. et al. An opposite pattern of selection of a single T cell antigen receptor in the thymus and among intraepithelial lymphocytes. J. Exp. Med. 188, 255–265 (1998).
Guehler, S. R., Bluestone, J. A. & Barrett, T. A. Immune deviation of 2C transgenic intraepithelial lymphocytes in antigen-bearing hosts. J. Exp. Med. 184, 493–503 (1996).
Guy-Grand, D. et al. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Exp. Med. 173, 471–481 (1991).
Lin, T. et al. CD3−CD8+ intestinal intraepithelial lymphocytes (IEL) and the extrathymic development of IEL. Eur. J. Immunol. 24, 1080–1087 (1994).
Rocha, B., Vassalli, P. & Guy-Grand, D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J. Exp. Med. 180, 681–686 (1994).
Lefrancois, L. & Olson, S. Reconstitution of the extrathymic intestinal T cell compartment in the absence of irradiation. J. Immunol. 159, 538–541 (1997).
Lambolez, F. et al. Characterization of T cell differentiation in the murine gut. J. Exp. Med. 195, 437–449 (2002).
Lin, T. et al. Thymus ontogeny and the development of TCRαβ intestinal intraepithelial lymphocytes. Cell. Immunol. 171, 132–139 (1996).
Lin, T., Matsuzaki, G., Kenai, H., Nakamura, T. & Nomoto, K. Thymus influences the development of extrathymically derived intestinal intraepithelial lymphocytes. Eur. J. Immunol. 23, 1968–1974 (1993).
Lefrancois, L. & Olson, S. A novel pathway of thymus-directed T lymphocyte maturation. J. Immunol. 153, 987–995 (1994).
Lin, T., Matsuzaki, G., Kenai, H. & Nomoto, K. Progenies of fetal thymocytes are the major source of CD4−CD8+αα intestinal intraepithelial lymphocytes early in ontogeny. Eur. J. Immunol. 24, 1785–1791 (1994).
Leishman, A. J. et al. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity 16, 355–364 (2002).
Yamagata, T., Mathis, D. & Benoist, C. Self-reactivity in thymic double-positive cells commits cells to a CD8 αα lineage with characteristics of innate immune cells. Nature Immunol. 5, 597–605 (2004).
Mao, X., Fujiwara, Y., Chapdelaine, A., Yang, H. & Orkin, S. H. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood 97, 324–326 (2001).
Guy-Grand, D. et al. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J. Exp. Med. 197, 333–341 (2003).
MacDonald, H. R., Radtke, F. & Wilson, A. T cell fate specification and αβ/γδ lineage commitment. Curr. Opin. Immunol. 13, 219–224 (2001).
Silva-Santos, B., Pennington, D. J. & Hayday, A. C. Lymphotoxin-mediated regulation of γδ cell differentiation by αβ T cell progenitors. Science 307, 925–928 (2005).
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Yoshida, H. et al. IL-7 receptor α+ CD3− cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 11, 643–655 (1999).
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M. H. & Pfeffer, K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Koni, P. A. et al. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity 6, 491–500 (1997).
Alimzhanov, M. B. et al. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc. Natl Acad. Sci. USA 94, 9302–9307 (1997).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17, 823–833 (2002).
Taylor, R. T., Lugering, A., Newell, K. A. & Williams, I. R. Intestinal cryptopatch formation in mice requires lymphotoxin α and the lymphotoxin β receptor. J. Immunol. 173, 7183–7189 (2004).
Adachi, S. et al. Essential role of IL-7 receptor α in the formation of Peyer's patch anlage. Int. Immunol. 10, 1–6 (1998).
Luther, S. A., Ansel, K. M. & Cyster, J. G. Overlapping roles of CXCL13, interleukin 7 receptor α and CCR7 ligands in lymph node development. J. Exp. Med. 197, 1191–1198 (2003).
Adachi, S., Yoshida, H., Kataoka, H. & Nishikawa, S. Three distinctive steps in Peyer's patch formation of murine embryo. Int. Immunol. 9, 507–514 (1997).
Honda, K. et al. Molecular basis for hematopoietic/ mesenchymal interaction during initiation of Peyer's patch organogenesis. J. Exp. Med. 193, 621–630 (2001).
Murray, A. M., Simm, B. & Beagley, K. W. Cytokine gene expression in murine fetal intestine: potential for extrathymic T cell development. Cytokine 10, 337–345 (1998).
Nishikawa, S., Honda, K., Vieira, P. & Yoshida, H. Organogenesis of peripheral lymphoid organs. Immunol. Rev. 195, 72–80 (2003).
Fujihashi, K. et al. Interleukin 2 (IL-2) and interleukin 7 (IL-7) reciprocally induce IL-7 and IL-2 receptors on γδ T-cell receptor-positive intraepithelial lymphocytes. Proc. Natl Acad. Sci. USA 93, 3613–3618 (1996).
Spahn, T. W. et al. Induction of colitis in mice deficient of Peyer's patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am. J. Pathol. 161, 2273–2282 (2002).
Kaiserling, E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology 34, 22–29 (2001).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Revy, P. et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000).
Yamamoto, M. et al. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J. Immunol. 173, 762–769 (2004).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Cario, E. & Podolsky, D. K. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68, 7010–7017 (2000).
Ortega-Cava, C. F. et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 170, 3977–3985 (2003).
Abreu, M. T. et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616 (2001).
Monteiro, R. C. & Van De Winkel, J. G. IgA Fc receptors. Annu. Rev. Immunol. 21, 177–204 (2003).
Ardavin, C. Origin, precursors and differentiation of mouse dendritic cells. Nature Rev. Immunol. 3, 582–590 (2003).
Papadakis, K. A. & Targan, S. R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 51, 289–298 (2000).
Brenner, O. et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc. Natl Acad. Sci. USA 101, 16016–16021 (2004).
Carlsen, H. S., Baekkevold, E. S., Johansen, F. E., Haraldsen, G. & Brandtzaeg, P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut 51, 364–371 (2002).
Gommerman, J. L. & Browning, J. L. Lymphotoxin/LIGHT, lymphoid microenvironments and autoimmune disease. Nature Rev. Immunol. 3, 642–655 (2003).
Tracey, K. J. & Cerami, A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 45, 491–503 (1994).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Sansonetti, P. J. War and peace at mucosal surfaces. Nature Rev. Immunol. 4, 953–964 (2004).
Mayer, L. & Shao, L. Therapeutic potential of oral tolerance. Nature Rev. Immunol. 4, 407–419 (2004).
Wu, H. Y. & Weiner, H. L. Oral tolerance. Immunol. Res. 28, 265–284 (2003).
Ruddle, N. H. Lymphoid neo-organogenesis: lymphotoxin's role in inflammation and development. Immunol. Res. 19, 119–125 (1999).
Hjelmstrom, P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J. Leukoc. Biol. 69, 331–339 (2001).
Moyron-Quiroz, J. E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nature Med. 10, 927–934 (2004).
Jacob, E., Baker, S. J. & Swaminathan, S. P. 'M' cells in the follicle-associated epithelium of the human colon. Histopathology 11, 941–952 (1987).
O'Leary, A. D. & Sweeney, E. C. Lymphoglandular complexes of the colon: structure and distribution. Histopathology 10, 267–283 (1986).
Guy-Grand, D., DiSanto, J. P., Henchoz, P., Malassis-Seris, M. & Vassalli, P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, TNF) in the induction of epithelial cell death and renewal. Eur. J. Immunol. 28, 730–744 (1998).
Hayday, A., Theodoridis, E., Ramsburg, E. & Shires, J. Intraepithelial lymphocytes: exploring the third way in immunology. Nature Immunol. 2, 997–1003 (2001).
Cheroutre, H. & Madakamutil, L. Acquired and natural memory T cells join forces at the mucosal front line. Nature Rev. Immunol. 4, 290–300 (2004).
Huang, F. P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Fleeton, M. N. et al. Peyer's patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J. Exp. Med. 200, 235–245 (2004).
Mebius, R. E., Streeter, P. R., Michie, S., Butcher, E. C. & Weissman, I. L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+CD3− cells to colonize lymph nodes. Proc. Natl Acad. Sci. USA 93, 11019–11024 (1996).
Mebius, R. E. et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol. 166, 6593–6601 (2001).
Yoshida, H. et al. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167, 2511–2521 (2001).
Acknowledgements
I thank R. Newberry, R. Mebius, N. Ruddle, M. Poles, D. Guy-Grand and P. Vassalli for discussion and critical reading of the manuscript. I also thank M. Tsuji for constant support and many debates, and D. Littman for introducing me to ROR-γt.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- ANTI-MESENTERIC BORDER
-
The mesentery is the membrane that attaches the small intestine to the back of the abdominal cavity. It also contains vessels that carry lymph and blood towards and away from the intestine. The anti-mesenteric border of the intestine is opposite to where the mesentery is attached.
- B-CELL FOLLICLE
-
An aggregate of B cells in lymphoid tissues. It contains naive B cells, as well as activated, proliferating and maturing B cells in germinal centres. B-cell follicles are contiguous with T-cell zones.
- COLONIC PATCHES
-
Lymphoid tissues that are similar to Peyer's patches and are present in the mucosa of the colon. Owing to the thick colonic muscle layer, colonic patches are not readily visible at the surface of the colon.
- CRYPTS
-
Crypts are tubular invaginations of the intestinal epithelium. At the base of the crypts, there are Paneth cells, which produce bactericidal defensins, and stem cells, which continuously divide and are the source of all intestinal epithelial cells. Contiguous with the crypts are the villi. These are projections into the lumen that have an outer layer that mainly consists of mature, absorptive enterocytes but also contains mucus-secreting goblet cells.
- FOLLICLE-ASSOCIATED EPITHELIUM (FAE).
-
The epithelium that overlies mucosal lymphoid tissues, such as the Peyer's patches and the isolated lymphoid follicles in the intestine. Lymphoid tissues induce the differentiation of normal intestinal epithelium into FAE, which is specialized in antigen capture and transport.
- INTRAEPITHELIAL LYMPHOCYTES (IELs).
-
Intraepithelial lymphocytes are T cells that reside on the basolateral side of the intestinal epithelium. They express either an αβ-TCR (T-cell receptor) or a γδ-TCR, as well as the CD8αα homodimer. This is in contrast to most conventional CD8+ T cells, which express the CD8αβ heterodimer. CD8αα is a ligand of the non-classical MHC class I molecule thymus leukaemia antigen (TL), which is expressed by the intestinal epithelium.
- MICROFOLD CELLS (M cells).
-
Differentiated epithelial cells that are present in follicle-associated epithelium. They transport antigens from the intestinal lumen into lymphoid tissues. Mucosal lymphoid tissues induce the differentiation of epithelial cells into M cells.
- PEYER'S PATCHES
-
Protruding lymphoid tissues that are present in the mucosa of the small intestine. In mice, they are composed of five to seven closely packed B-cell follicles. They are named after Johann Conrad Peyer (1653–1712), a Swiss anatomist.
Rights and permissions
About this article
Cite this article
Eberl, G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway?. Nat Rev Immunol 5, 413–420 (2005). https://doi.org/10.1038/nri1600
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1600
This article is cited by
-
An integrin αEβ7-dependent mechanism of IgA transcytosis requires direct plasma cell contact with intestinal epithelium
Mucosal Immunology (2021)
-
A review of metabolic potential of human gut microbiome in human nutrition
Archives of Microbiology (2018)
-
Dynamics of HIV infection in lymphoid tissue network
Journal of Mathematical Biology (2016)
-
Toolbox murders: putting genes in their epigenetic and ecological contexts
Biology & Philosophy (2016)
-
Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine
Nature Immunology (2015)