Key Points
-
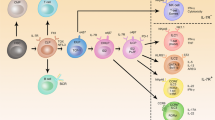
For the formation of lymph nodes and Peyer's patches, lymphotoxin (LT)α1β2-mediated signalling through the lymphotoxin-β receptor (LTβR) is essential. LTα1β2 is expressed by haematopoietic cells, whereas LTβR is present on stromal cells.
-
The cells that express LTα1β2 are CD3−CD4+CD45+ cells. In the absence of these cells, lymph nodes and Peyer's patches can not form.
-
The cells that express LTβR are further characterized by expression of vascular-cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1). These cells produce the chemokines CXCL13 and CCL19.
-
Binding of CD3−CD4+CD45+ cells to stromal cells requires activation of the β1 integrin on CD3−CD4+CD45+ cells. This allows active α4β1 integrin binding to VCAM1 on stromal cells, after which LTα1β2-mediated signalling through LTβR can take place.
-
After LTβR signalling, two NF-κB pathways are activated. Activation of the classical NF-κB pathway is required for the induction of VCAM1, whereas activation of the alternative pathway, which requires NF-κB-inducing kinase (NIK), is necessary for the induction of chemokines (that is, CXCL13).
-
The chemokine CXCL13 is essential for lymphoid organogenesis. Its role in this process could be manifold, as it might induce activation of β1 integrin, attract CD3−CD4+CD45+ cells and be involved in the induction of LTα1β2 expression.
-
Cell clustering of haematopoietic cells and stromal cells is essential for lymphoid organogenesis. It allows cellular signalling required for further development of the structure.
-
Throughout the process of lymphoid organogenesis, LTα1β2 is important, because it is required for the formation, as well as the organization, of the lymphoid organs. LTα1β2 could have a similar role in establishing organized tertiary lymphoid structures during inflammatory reactions.
Abstract
The development of lymphoid organs depends on the correct expression of several molecules within a defined timeframe during ontogeny. Although this is an extremely complex process, with each secondary lymphoid tissue requiring subtly different signals, a common framework for lymphoid development is beginning to emerge. Drawing on studies of lymph nodes, Peyer's patches and nasal-associated lymphoid tissue, an integrative model of lymphoid-tissue development, involving adhesion molecules, cytokines and chemokines, which emphasizes the role of interactions between CD3−CD4+CD45+ 'inducer' cells and VCAM1+ICAM1+ stromal 'organizer' cells is presented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hjelmstrom, P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J. Leukocyte Biol. 69, 331–339 (2001).
Takemura, S. et al. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167, 1072–1080 (2001).
Armengol, M. P. et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am. J. Pathol. 159, 861–873 (2001).
Amft, N. et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren's syndrome. Arthritis Rheum. 44, 2633–2641 (2001).
Salomonsson, S. et al. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren's syndrome. Scand. J. Immunol. 55, 336–342 (2002).
Sabin, F. R. The lymphatic system in human embryos, with a consideration of the morphology of the system as a whole. Am. J. Anat. 9, 43–91 (1909).
Sabin, F. R. Further evidence on the origin of the lymphatic endothelium from the endothelium of the blood vascular system. Anat. Rec. 2, 46–55 (1908).
Sabin, F. R. On the developement of the superficial lymphatics in the skin of the pig. Am. J. Anat. 3, 183–195 (1904).
Sabin, F. R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic ducts in the pig. Am. J. Anat. 1, 367–389 (1902).
Bailey, R. P. & Weiss, L. Ontogeny of human fetal lymph nodes. Am. J. Anat. 142, 15–27 (1975).
Eikelenboom, P., Nassy, J. J., Post, J., Versteeg, J. C. & Langevoort, H. L. The histogenesis of lymph nodes in rat and rabbit. Anat. Rec. 190, 201–215 (1978).
Wigle, J. T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999). This papers confirms Sabin's theory (references 6–8) on the formation of lymph sacs.
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17, 823–833 (2002).
Yoshida, H. et al. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167, 2511–2521 (2001).
Honda, K. et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J. Exp. Med. 193, 621–630 (2001). The first paper to characterize the stromal cells that are involved in Peyer's-patch development.
Scheu, S. et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin-β in mesenteric lymph node genesis. J. Exp. Med. 195, 1613–1624 (2002).
Rennert, P. D., Browning, J. L., Mebius, R., Mackay, F. & Hochman, P. S. Surface lymphotoxin-α/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184, 1999–2006 (1996).
Koni, P. A. et al. Distinct roles in lymphoid organogenesis for lymphotoxins-α and -β revealed in lymphotoxin-β-deficient mice. Immunity 6, 491–500 (1997).
Alimzhanov, M. B. et al. Abnormal development of secondary lymphoid tissues in lymphotoxin-β-deficient mice. Proc. Natl Acad. Sci. USA 94, 9302–9307 (1997).
Banks, T. A. et al. Lymphotoxin-α-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J. Immunol. 155, 1685–1693 (1995).
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M. H. & Pfeffer, K. The lymphotoxin-β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994). This was the first paper to describe and emphasize defective lymphoid-organ development in mutant mice.
Kuprash, D. V. et al. TNF and lymphotoxin-β cooperate in the maintenance of secondary lymphoid tissue microarchitecture but not in the development of lymph nodes. J. Immunol. 163, 6575–6580 (1999).
Rennert, P. D., James, D., Mackay, F., Browning, J. L. & Hochman, P. S. Lymph node genesis is induced by signaling through the lymphotoxin-β receptor. Immunity 9, 71–79 (1998). This work showed the crucial involvement of lymphotoxin-β receptor (LTβR) triggering for lymph-node development.
Matsumoto, M. et al. Involvement of distinct cellular compartments in the abnormal lymphoid organogenesis in lymphotoxin-α-deficient mice and alymphoplasia (aly) mice defined by the chimeric analysis. J. Immunol. 163, 1584–1591 (1999).
Dejardin, E. et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 17, 525–535 (2002). This paper shows that two NF-κB pathways are turned on after LTβR triggering and describes which genes are induced downstream from these pathways.
Ngo, V. N. et al. Lymphotoxin-α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999). This paper shows that LTα 1 β 2 and tumour-necrosis factor (TNF) are required for the expression of lymphoid chemokines that mediate compartmentalization in lymphoid organs.
Cuff, C. A., Sacca, R. & Ruddle, N. H. Differential induction of adhesion molecule and chemokine expression by LTα3 and LTαβ in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J. Immunol. 162, 5965–5972 (1999).
Yilmaz, B. Z., Weih, D. S., Sivakumar, V. & Weih, F. RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J. 22, 121–130 (2003).
Dougall, W. C. et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 13, 2412–2424 (1999).
Kim, D. et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J. Exp. Med. 192, 1467–1478 (2000).
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Harmsen, A. et al. Cutting edge: organogenesis of nasal-associated lymphoid tissue (NALT) occurs independently of lymphotoxin-α (LT-α) and retinoic acid receptor-related orphan receptor-γ, but the organization of NALT is LT-α dependent. J. Immunol. 168, 986–990 (2002).
Naito, A. et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4, 353–362 (1999).
Georgopoulos, K. et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 79, 143–156 (1994).
Wang, J. H. et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 5, 537–549 (1996).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Kurebayashi, S. et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl Acad. Sci. USA 97, 10132–10137 (2000).
Yokota, Y. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706 (1999). This paper, together with reference 32, shows for the first time the complete absence of CD3−CD4+CD45+ cells in mice with defective lymphoid organogenesis.
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor-γ chain. Immunity 2, 223–238 (1995).
Park, S. Y. et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity 3, 771–782 (1995).
Freeden-Jeffry, U. V. et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181, 1519–1526 (1995).
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000). This paper describes a positive-feedback loop, in which CXCL13-mediated CXCR5 triggering leads to increased LTα 1 β 2 expression, which signals through LTβR, inducing further production of CXCL13.
Gunn, M. D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Koni, P. A. & Flavell, R. A. A role for tumor necrosis factor receptor type 1 in gut-associated lymphoid tissue development: genetic evidence of synergism with lymphotoxin-β. J. Exp. Med. 187, 1977–1983 (1998).
Forster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Adachi, S., Yoshida, H., Kataoka, H. & Nishikawa, S. Three distinctive steps in Peyer's patch formation of murine embryo. Int. Immunol. 9, 507–514 (1997). This paper was the first to distinguish three steps in Peyer's-patch development, which has been helpful for further understanding of lymphoid organogenesis.
Yoshida, H. et al. IL-7 receptor-α+ CD3− cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 11, 643–655 (1999).
Adachi, S. et al. Essential role of IL-7 receptor α in the formation of Peyer's patch anlage. Int. Immunol. 10, 1–6 (1998).
Laky, K. et al. Enterocyte expression of interleukin 7 induces development of γδ T cells and Peyer's patches. J. Exp. Med. 191, 1569–1580 (2000).
Pasparakis, M. et al. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl Acad. Sci. USA 94, 6319–6323 (1997).
Neumann, B., Luz, A., Pfeffer, K. & Holzmann, B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J. Exp. Med. 184, 259–264 (1996).
Korner, H. et al. Distinct roles for lymphotoxin-α and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 27, 2600–2609 (1997).
Fukuyama, S. et al. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3−CD4+CD45+ cells. Immunity 17, 31–40 (2002). This was the first paper to show the inductive role of CD3−CD4+CD45+ cells in lymphoid organogenesis.
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997). This was the first paper to identify the expression of LTα 1 β 2 on CD3−CD4+CD45+ cells.
Finke, D., Acha-Orbea, H., Mattis, A., Lipp, M. & Kraehenbuhl, J. CD4+CD3− cells induce Peyer's patch development. Role of α4β1 integrin activation by CXCR5. Immunity 17, 363–373 (2002). This paper shows the inductive role of CD3−CD4+CD45+ cells in Peyer's-patch development and provides a further model for the role of CXCR5, α 4 β 1 integrin and VCAM1 in lymphoid organogenesis.
Browning, J. L. & French, L. E. Visualization of lymphotoxin-β and lymphotoxin-β receptor expression in mouse embryos. J. Immunol. 168, 5079–5087 (2002).
Cuff, C. A. et al. Lymphotoxin-α3 induces chemokines and adhesion molecules: insight into the role of LT-α in inflammation and lymphoid organ development. J. Immunol. 161, 6853–6860 (1998).
Luther, S. A., Lopez, T., Bai, W., Hanahan, D. & Cyster, J. G. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 12, 471–481 (2000).
Chen, S. C. et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J. Immunol. 168, 1001–1008 (2002).
Fan, L., Reilly, C. R., Luo, Y., Dorf, M. E. & Lo, D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J. Immunol. 164, 3955–3959 (2000).
Luther, S. A. et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433 (2002).
Nakano, H. et al. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 27, 215–221 (1997).
Gunn, M. D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Vassileva, G. et al. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 190, 1183–1188 (1999).
Luther, S. A., Tang, H. L., Hyman, P. L., Farr, A. G. & Cyster, J. G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl Acad. Sci. USA 97, 12694–12699 (2000).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Mebius, R. E. et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol. 166, 6593–6601 (2001).
Matsumoto, K., Yoshitomi, H., Rossant, J. & Zaret, K. S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559–563 (2001).
Lammert, E., Cleaver, O. & Melton, D. Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 (2001).
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Gonzalez, M., Mackay, F., Browning, J. L., Kosco-Vilbois, M. H. & Noelle, R. J. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 187, 997–1007 (1998).
Fu, Y. X., Huang, G., Wang, Y. & Chaplin, D. D. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α-dependent fashion. J. Exp. Med. 187, 1009–1018 (1998).
Endres, R. et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin β receptor by radioresistant stromal cells and of lymphotoxin β and tumor necrosis factor by B cells. J. Exp. Med. 189, 159–168 (1999).
Tumanov, A. et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 17, 239–250 (2002).
Hashi, H. et al. Compartmentalization of Peyer's patch anlagen before lymphocyte entry. J. Immunol. 166, 3702–3709 (2001).
Matsushima, A. et al. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 193, 631–636 (2001).
Smith, C. et al. NF-κB-inducing kinase is dispensable for activation of NF-κB in inflammatory settings but essential for lymphotoxin β receptor activation of NF-κB in primary human fibroblasts. J. Immunol. 167, 5895–5903 (2001).
Yin, L. et al. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291, 2162–2165 (2001).
Miyawaki, S. et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24, 429–434 (1994).
Shinkura, R. et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nature Genet. 22, 74–77 (1999).
Paxian, S. et al. Abnormal organogenesis of Peyer's patches in mice deficient for NF-κB1, NF-κB2, and Bcl-3. Gastroenterology 122, 1853–1868 (2002).
Weih, F. et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell 80, 331–340 (1995).
Sha, W. C., Liou, H. C., Tuomanen, E. I. & Baltimore, D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80, 321–330 (1995).
Alcamo, E. et al. Requirement for the NF-κB family member RelA in the development of secondary lymphoid organs. J. Exp. Med. 195, 233–244 (2002).
Weih, D. S., Yilmaz, Z. B. & Weih, F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J. Immunol. 167, 1909–1919 (2001).
Acknowledgements
I thank T. Cupedo, T. O'Toole and G. Kraal for critically reading the manuscript and for their useful suggestions. The work in my laboratory is supported by grants from the Netherlands Organisation for Scientific Research.
Author information
Authors and Affiliations
Glossary
- LYMPHOTOXIN
-
(LT). This protein belongs to the tumour-necrosis factor family and can be produced as a secreted homotrimer, LTα3, or as a membrane-bound heterotrimer, LTα1β2. The heterotrimer LTα1β2 binds to the lymphotoxin-β receptor (LTβR).
- ALYMPHOPLASIA
-
(aly). A spontaneous mutation characterized by the systemic absence of lymph nodes and Peyer's patches. The gene responsible for this phenotype was shown to encode nuclear factor-κB-inducing kinase (NIK).
- IKAROS
-
This gene encodes a family of zinc-finger transcription factors that regulate transcription required for the development of all lymphoid lineages, as well as lymph nodes and Peyer's patches.
- ID2
-
A helix-loop-helix protein that can inhibit transcription factors with a basic helix-loop-helix motif. Besides its role in lymphoid organogenesis, Id2 is required for the generation of natural killer cells.
- RETINOID-RELATED ORPHAN RECEPTOR γ
-
(RORγ). An orphan nuclear hormone receptor that regulates the survival of CD4+CD8+ thymocytes and is essential for the development of lymph nodes and Peyer's patches.
- LYMPHOID CHEMOKINES
-
These are constitutively expressed in lymphoid tissues and mediate the formation and maintenance of micro-domains in lymphoid organs.
- HIGH ENDOTHELIAL VENULES
-
(HEVs). Specialized venules found in lymphoid tissues, which are the site of entry for lymphocytes from the bloodstream into lymph nodes and Peyer's patches.
- PAUCITY OF LYMPH NODE T CELLS
-
(plt). A mutation that leads to the loss of expression of the chemokines CCL19 and CCL21 in lymphoid organs, resulting in disturbed migration of CCR7-expressing T cells and mature dendritic cells.
- FOLLICULAR DENDRITIC CELLS
-
(FDCs). These stromal cells present immune complexes to B cells in the B-cell follicles.
Rights and permissions
About this article
Cite this article
Mebius, R. Organogenesis of lymphoid tissues. Nat Rev Immunol 3, 292–303 (2003). https://doi.org/10.1038/nri1054
Issue Date:
DOI: https://doi.org/10.1038/nri1054
This article is cited by
-
Transcription factors: key regulatory targets of vascular smooth muscle cell in atherosclerosis
Molecular Medicine (2023)
-
Lymphoid tissue inducer cells in cancer: a potential therapeutic target
Molecular and Cellular Biochemistry (2023)
-
CP-25 alleviates antigen-induced experimental Sjögren's syndrome in mice by inhibiting JAK1-STAT1/2-CXCL13 signaling and interfering with B-cell migration
Laboratory Investigation (2021)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
Lymph node stromal cells: cartographers of the immune system
Nature Immunology (2020)