Abstract
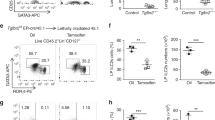
The location of embryonic lymph node development is determined by the initial clustering of lymphoid tissue–inducer (LTi) cells. Here we demonstrate that both the chemokine CXCL13 and the chemokine CCL21 attracted LTi cells at embryonic days 12.5–14.5 and that initial clustering depended exclusively on CXCL13. Retinoic acid (RA) induced early CXCL13 expression in stromal organizer cells independently of lymphotoxin signaling. Notably, neurons adjacent to the lymph node anlagen expressed enzymes essential for RA synthesis. Furthermore, stimulation of parasymphathetic neural output in adults led to RA receptor (RAR)-dependent induction of CXCL13 in the gut. Therefore, our data show that the initiation of lymph node development is controlled by RA-mediated expression of CXCL13 and suggest that RA may be provided by adjacent neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mebius, R.E., Streeter, P.R., Michie, S., Butcher, E.C. & Weissman, I.L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+CD3− cells to colonize lymph nodes. Proc. Natl. Acad. Sci. USA 93, 11019–11024 (1996).
Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Cupedo, T. & Mebius, R.E. Cellular interactions in lymph node development. J. Immunol. 174, 21–25 (2005).
Yoshida, H. et al. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167, 2511–2521 (2001).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Mebius, R.E., Rennert, P. & Weissman, I.L. Developing lymph nodes collect CD4+CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Boos, M.D., Yokota, Y., Eberl, G. & Kee, B.L. Mature natural killer cell and lymphoid tissue–inducing cell development requires Id2–mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130 (2007).
Yoshida, H. et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17, 823–833 (2002).
Meier, D. et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654 (2007).
Cupedo, T. et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J. Immunol. 173, 2968–2975 (2004).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Rennert, P.D., James, D., Mackay, F., Browning, J.L. & Hochman, P.S. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity 9, 71–79 (1998).
Vondenhoff, M.F. et al. LTβR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J. Immunol. 182, 5439–5445 (2009).
Luther, S.A. et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169, 424–433 (2002).
Luther, S.A., Ansel, K.M. & Cyster, J.G. Overlapping roles of CXCL13, interleukin 7 receptor α and CCR7 ligands in lymph node development. J. Exp. Med. 197, 1191–1198 (2003).
Ansel, K.M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Ohl, L. et al. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 197, 1199–1204 (2003).
Veiga–Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature 446, 547–551 (2007).
Niederreither, K. et al. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 130, 2525–2534 (2003).
Vermot, J. et al. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development 132, 1611–1621 (2005).
Niederreither, K. & Dolle, P. Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553 (2008).
Mora, J.R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006).
Johansson–Lindbom, B. & Agace, W.W. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 215, 226–242 (2007).
Mebius, R.E. Vitamins in control of lymphocyte migration. Nat. Immunol. 8, 229–230 (2007).
Hammerschmidt, S.I. et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205, 2483–2490 (2008).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Mebius, R.E. et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol. 166, 6593–6601 (2001).
Vondenhoff, M.F. et al. Separation of splenic red and white pulp occurs before birth in a LTαβ-independent manner. J. Leukoc. Biol. 84, 152–161 (2008).
Schug, T.T., Berry, D.C., Shaw, N.S., Travis, S.N. & Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129, 723–733 (2007).
Li, Y., Hashimoto, Y., Agadir, A., Kagechika, H. & Zhang, X. Identification of a novel class of retinoic acid receptor β-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J. Biol. Chem. 274, 15360–15366 (1999).
Svensson, M. et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol 1, 38–48 (2008).
Niederreither, K., Subbarayan, V., Dolle, P. & Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444–448 (1999).
Niederreither, K. et al. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128, 1019–1031 (2001).
de Jonge, W.J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2–STAT3 signaling pathway. Nat. Immunol. 6, 844–851 (2005).
Borovikova, L.V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Forster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Van Der Zanden, E.P., Boeckxstaens, G.E. & de Jonge, W.J. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol. Motil. 21, 6–17 (2009).
Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 (2007).
Magliozzi, R., Columba-Cabezas, S., Serafini, B. & Aloisi, F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 148, 11–23 (2004).
Wengner, A.M. et al. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 56, 3271–3283 (2007).
Meraouna, A. et al. The chemokine CXCL13 is a key molecule in autoimmune myasthenia gravis. Blood 108, 432–440 (2006).
Steinmetz, O.M. et al. BCA-1/CXCL13 expression is associated with CXCR5-positive B-cell cluster formation in acute renal transplant rejection. Kidney Int. 67, 1616–1621 (2005).
Bagaeva, L.V., Rao, P., Powers, J.M. & Segal, B.M. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J. Immunol. 176, 7676–7685 (2006).
Grimsholm, O., Guo, Y., Ny, T. & Forsgren, S. Expression patterns of neurotrophins and neurotrophin receptors in articular chondrocytes and inflammatory infiltrates in knee joint arthritis. Cells Tissues Organs 188, 299–309 (2008).
Sugiura, H., Omoto, M., Hirota, Y., Danno, K. & Uehara, M. Density and fine structure of peripheral nerves in various skin lesions of atopic dermatitis. Arch. Dermatol. Res. 289, 125–131 (1997).
Batchelor, P.E., Wills, T.E., Hewa, A.P., Porritt, M.J. & Howells, D.W. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res. 1209, 49–56 (2008).
Manzo, A. et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur. J. Immunol. 35, 1347–1359 (2005).
Moyron–Quiroz, J.E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 0034.1–0034.11 (2002).
Cupedo, T. et al. Initiation of cellular organization in lymph nodes is regulated by non–B cell–derived signals and is not dependent on CXC chemokine ligand 13. J. Immunol. 173, 4889–4896 (2004).
Acknowledgements
We thank animal caretakers for care of the animals; G. Kraal and T. Geijtenbeek for critically reading the manuscript; R. Molenaar for help; H. Kalay for labeling antibodies; D. Littman (Skirball Institute of Biomolecular Medicine), S. Nishikawa (Riken Center for Developmental Biology) and K. van Gisbergen (Academic Medical Center, Amsterdam) for antibodies; and J. van der Meulen (Centraal Bureau voor de Statistiek, The Netherlands) for help with statistical analysis. Supported by the US National Institutes of Health (HL69409 and AI072689 to T.D.R.) and the Netherlands Organization for Scientific Research (VIDI grant 016.096.310 to W.J.d.J.; VICI grant 918.56.612 to R.E.M. and S.A.v.d.P.; and Genomics grant 050-10-120 to R.E.M. and M.F.V.).
Author information
Authors and Affiliations
Contributions
S.A.v.d.P., B.J.O., M.F.V., G.G., M.G., W.J.d.J. and P.B. did the experiments and data analysis; K.K. and T.D.R. provided the Cxcl13−/− embryos; U.E.H. and M.L. provided the Cxcr5−/− embryos; K.N. provided the Raldh2−/− embryos; R.B., K.S. and W.W.A. provided the DR5 embryos; S.A.v.d.P. and R.E.M. designed the experiments; S.A.v.d.P., T.D.R., and R.E.M. wrote the manuscript; and R.E.M. directed the study.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–2 (PDF 575 kb)
Rights and permissions
About this article
Cite this article
van de Pavert, S., Olivier, B., Goverse, G. et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol 10, 1193–1199 (2009). https://doi.org/10.1038/ni.1789
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1789
This article is cited by
-
B cell development and antibody responses in human immune system mice: current status and future perspective
Science China Life Sciences (2024)
-
Immune subset-committed proliferating cells populate the human foetal intestine throughout the second trimester of gestation
Nature Communications (2023)
-
Tertiary Lymphoid Structures Are Associated with a Favorable Prognosis in High-Grade Serous Ovarian Cancer Patients
Reproductive Sciences (2023)
-
Lymph node stromal cells: cartographers of the immune system
Nature Immunology (2020)
-
YAP/TAZ direct commitment and maturation of lymph node fibroblastic reticular cells
Nature Communications (2020)