Abstract
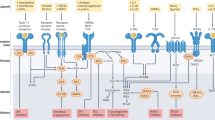
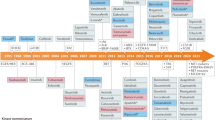
Protein kinases, which serve critical functions in signaling pathways in all cells, are popular therapeutic targets. At present, eight kinase inhibitors have been approved in the United States, each of which shows nanomolar potency. Although the initial goal was to generate inhibitors with a high degree of selectivity, recent experience has revealed that many of these approved compounds target more than one kinase. Surprisingly, this promiscuity is less problematic than one would have imagined; indeed, it opens new therapeutic opportunities. In this Perspective, we discuss the present status of Janus kinase inhibitors—a new class of immunosuppressive drugs—and the advantages and disadvantages of selectively inhibiting this class of kinase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fischer, E.H. & Krebs, E.G. Relationship of structure to function of muscle phosphorylase. Fed. Proc. 25, 1511–1520 (1966).
Venter, J.C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Manning, G., Whyte, D.B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Hunter, T. A thousand and one protein kinases. Cell 50, 823–829 (1987).
Pawson, T. Protein-tyrosine kinases. Getting down to specifics. Nature 373, 477–478 (1995).
Pawson, T. Protein modules and signalling networks. Nature 373, 573–580 (1995).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science 289, 1938–1942 (2000).
Daley, G.Q., Van Etten, R.A. & Baltimore, D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247, 824–830 (1990).
Druker, B.J. et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2, 561–566 (1996).
O'Brien, S.G. et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 (2003).
Druker, B.J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Geyer, C.E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Karaman, M.W. et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 26, 127–132 (2008).
Cools, J. et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 348, 1201–1214 (2003).
Simon, M.P. et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat. Genet. 15, 95–98 (1997).
Baroni, S.S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Louvet, C. et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 105, 18895–18900 (2008).
Gorre, M.E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876–880 (2001).
Azam, M., Seeliger, M.A., Gray, N.S., Kuriyan, J. & Daley, G.Q. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat. Struct. Mol. Biol. 15, 1109–1118 (2008).
Lee, J.C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Russell, S.M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Karaghiosoff, M. et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 13, 549–560 (2000).
Minegishi, Y. et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25, 745–755 (2006).
Duerr, R.H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Burton, P.R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Leonardi, C.L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Changelian, P.S. et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302, 875–878 (2003).
Kremer, J.M. et al. A randomized, double-blind placebo-controlled trial of 3 dose levels of CP-690,550 versus placebo in the treatment of active rheumatoid arthritis. Arthritis Rheum. 54, 4116 (2006).
Wilkinson, B., Chow, V., LaBadie, R., Zwillich, S.H. & Cohen, S. Co-administration of an oral JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis. Arthritis Rheum. 58, S297 (2008).
Chan, G., Cunshan, W., Boy, M., Chow, V. & Herron, J. Dose-dependent reduction in psoriasis severity as evidence of immunosuppressive activity of an oral Jak3 inhibitor in humans. Am. J. Transplant. 6, S87 (2006).
van Gurp, E. et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. Am. J. Transplant. 8, 1711–1718 (2008).
Parganas, E. et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 (1998).
Baxter, E.J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
James, C. et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 (2005).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 (2005).
Levine, R.L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 (2005).
Geron, I. et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell 13, 321–330 (2008).
Wernig, G. et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 13, 311–320 (2008).
Manshouri, T. et al. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 99, 1265–1273 (2008).
Williams, W. et al. A randomized placebo-controlled study of INCB018424, a selective Janus kinase1&2 (JAK1&2) inhibitor in rheumatoid arthritis (RA). Arthritis Rheum. 58, S431 (2008).
Weinblatt, M.E. et al. Treatment of rheumatoid arthritis with a syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 58, 3309–3318 (2008).
Engelman, J.A. et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 14, 1351–1356 (2008).
Apsel, B. et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Chem. Biol. 4, 691–699 (2008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The US National Institutes of Health and J.J.O. hold a patent related to Janus family kinases and identification of immune modulators, and have a Collaborative Research Agreement and Development Award with Pfizer.
Rights and permissions
About this article
Cite this article
Ghoreschi, K., Laurence, A. & O'Shea, J. Selectivity and therapeutic inhibition of kinases: to be or not to be?. Nat Immunol 10, 356–360 (2009). https://doi.org/10.1038/ni.1701
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1701
This article is cited by
-
Suppressor of cytokine signaling-1 mimetic peptides attenuate lymphocyte activation in the MRL/lpr mouse autoimmune model
Scientific Reports (2021)
-
Expression of JAK3, STAT2, STAT4, and STAT6 in pemphigus vulgaris
Immunologic Research (2020)
-
Pharmakologie der Januskinaseinhibitoren
Der Hautarzt (2019)
-
The use of novel selectivity metrics in kinase research
BMC Bioinformatics (2017)
-
Mechanisms and consequences of Jak–STAT signaling in the immune system
Nature Immunology (2017)