Abstract
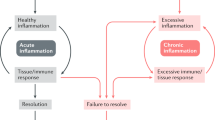
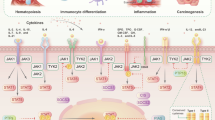
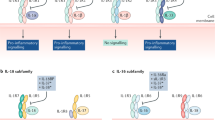
Signaling pathways enable cells to respond and adapt to environmental stimuli. For instance, extracellular ligands, such as proinflammatory cytokines or pathogen components, bind receptors on the surface of cells that trigger downstream signaling cascades driven by enzymes called kinases. Ultimately, kinases activate transcription factors that bind to DNA and alter the expression of target genes, the products of which allow the cell to respond to the initial stimulus. A variety of chronic inflammatory diseases are associated with altered cellular signaling. Some of the signal cascades that are involved in inflammation and autoimmunity include those mediated by mitogen-activated protein kinases, nuclear factor-κB, interferon regulatory factor and Toll-like receptors, NOD-like receptors and the inflammasome, and phosphatidylinositol-3-kinases. Understanding these intracellular pathways might lead to new approaches to the treatment of inflammatory disease, including the use of orally bioavailable small molecules that regulate cytokine function and production.
Key Points
-
Signal transduction is a communication system that provides information to the cell and regulates responses to environmental stress
-
Protein kinases that phosphorylate transcription factors and other molecules have a key role in signal transduction
-
Multiple signaling pathways, including those mediated by mitogen-activated protein kinases, nuclear factor-κB and others, activate immune responses and inflammation by regulating the expression of cytokines and other mediators
-
Signal transduction can potentially be targeted to treat immune-mediated diseases like rheumatoid arthritis and systemic lupus erythematosus
-
Selectivity of signal transduction inhibitors and potential adverse effects related to host defense and homeostasis represent major hurdles to drug development
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tas SW et al. (2005) Signal transduction pathways and transcription factors as therapeutic targets in inflammatory disease: towards innovative antirheumatic therapy. Curr Pharm Des 11: 581–611
Manning AM and Davis RJ (2003) Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov 2: 554–565
Inoue T et al. (2006) Regulation of JNK by MKK7 in fibroblast-like synoviocytes. Arthritis Rheum 54: 2127–2135
Chabaud-Riou M and Firestein GS (2004) Expression and activation of MKK3 and MKK6 in rheumatoid arthritis. Am J Pathol 164: 177–184
Inoue T et al. (2005) Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J Immunol 174: 4301–4306
Toh M et al. (2004) Expression of mitogen-activated protein kinase phosphatase 1, a negative regulator of the mitogen-activated protein kinases, in rheumatoid arthritis: up-regulation by interleukin-1beta and glucocorticoids. Arthritis Rheum 50: 3118–3128
Schett G et al. (2000) Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum 43: 2501–2512
Han Z et al. (1999) Jun N-terminal kinase in rheumatoid arthritis. J Pharmacol Exp Ther 291: 124–130
Han Z et al. (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108: 73–81
Badger A et al. (2000) Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum 43: 175–183
Nishikawa M et al. (2003) Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum 48: 2670–2681
Zwerina J et al. (2006) Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum 54: 463–472
Grammer A et al. (2004) Flow cytometric assessment of the signaling status of human B lymphocytes from normal and autoimmune individuals. Arthritis Res Ther 6: 28–38
Takahashi H et al. (2002) Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. J Dermatol Sci 30: 94–99
Zenz R et al. (2005) Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437: 369–375
Johansen C et al. (2005) The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol 152: 37–42
Hollenbach E et al. (2005) Inhibition of RICK/nuclear factor-kappa B and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem 280: 14981–14988
Escott K et al. (2000) Effect of the p38 kinase inhibitor, SB 203580, on allergic airway inflammation in the rat. Br J Pharmacol 131: 173–176
Aupperle K et al. (2001) NF-kappa B regulation by I kappa B kinase-2 in rheumatoid arthritis synoviocytes. J Immunol 166: 2705–2711
Tak PP et al. (2001) Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum 44: 1897–1907
Miagkov A et al. (1998) NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA 95: 13859–13864
Andreakos E et al. (2003) Heterogeneous requirement of IkappaB kinase 2 for inflammatory cytokine and matrix metalloproteinase production in rheumatoid arthritis: implications for therapy. Arthritis Rheum 48: 1901–1912
McIntyre KW et al. (2003) A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum 48: 2652–2659
Hammaker D et al. (2003) Signal transduction networks in rheumatoid arthritis. Ann Rheum Dis 62: 1186–1189
Diebold S et al. (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303: 1529–1531
Heil F et al. (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303: 1526–1529
Krieg A et al. (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374: 546–549
Crow M (2005) Interferon pathway activation in systemic lupus erythematosus. Curr Rheumatol Rep 7: 463–468
Radstake TR et al. (2004) Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum 50: 3856–3865
Seibl R et al. (2003) Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol 162: 1221–1227
van der Heijden IM et al. (2000) Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum 43: 593–598
Choe JY et al. (2003) Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med 197: 537–542
Kyburz D et al. (2003) Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum 48: 642–650
Seibl R et al. (2003) Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol 162: 1221–1227
Brentano F et al. (2005) RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum 52: 2656–2665
Sharma S et al. (2003) Triggering the interferon antiviral response through an IKK-related pathway. Science 300: 1148–1151
Fitzgerald KA et al. (2003) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4: 491–496
Sweeney S et al. (2007) Antiviral gene expression in rheumatoid arthritis: role of IKKepsilon and interferon regulatory factor 3. Arthritis Rheum 56: 743–752
Sweeney SE et al. (2005) Regulation of c-Jun phosphorylation by the I kappa B kinase-epsilon complex in fibroblast-like synoviocytes. J Immunol 174: 6424–6430
Baechler E et al. (2004) The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol 16: 801–807
Vallin H et al. (1999) Anti-double stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol 163: 6306–6313
Rönnblom L and Alm G (2001) A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med 194: F59–F63
Kaisho T and Akira S (2006) Toll-like receptor function and signaling. J Allergy Clin Immunol 117: 979–987
Anders HJ (2005) A Toll for lupus. Lupus 14: 417–422
Lenert PS (2006) Targeting Toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus. Arthritis Res Ther 8: 203
Christensen S et al. (2006) Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25: 417–428
Barrat F et al. (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 202: 1131–1139
Ronaghy A et al. (2002) Immunostimulatory DNA sequences influence the course of adjuvant arthritis. J Immunol 168: 51–56
Means T et al. (2005) Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 115: 407–417
Weyand C et al. (2005) Vascular dendritic cells in giant cell arteritis. Ann NY Acad Sci 1062: 195–208
Akira S et al. (2006) Pathogen recognition and innate immunity. Cell Immunol 124: 783–801
Shi Y et al. (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521
Martinon F et al. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241
Curnock A et al. (2002) Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology 105: 125–136
Okkenhaug K and Vanhaesebroeck B (2003) PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 3: 317–330
Barber D et al. (2005) PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 11: 933–935
Camps M et al. (2005) Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11: 936–943
Hayer S et al. (2006) Challenging the role of PI3K-gamma in arthritis. Arthritis Rheum 54 (Suppl): S599
Rommel C et al. (2007) PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7: 191–201
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sweeney, S., Firestein, G. Primer: signal transduction in rheumatic disease—a clinician's guide. Nat Rev Rheumatol 3, 651–660 (2007). https://doi.org/10.1038/ncprheum0631
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0631
This article is cited by
-
Mitochondria transfer restores fibroblasts-like synoviocytes (FLS) plasticity in LPS-induced, in vitro synovitis model
Cell Communication and Signaling (2022)
-
Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis
Arthritis Research & Therapy (2017)
-
Signal transducer and activator of transcription 5 is implicated in disease activity in adult and juvenile onset systemic lupus erythematosus
Clinical Rheumatology (2016)
-
Germinal center kinase-like kinase (GLK/MAP4K3) expression is increased in adult-onset Still's disease and may act as an activity marker
BMC Medicine (2012)
-
The Role of Raf Kinase Inhibitor Protein in Rheumatoid Fibroblast-like Synoviocytes Invasiveness and Cytokine and Matrix Metalloproteinase Expression
Inflammation (2012)