Abstract
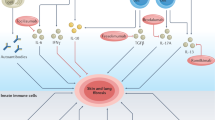
Systemic sclerosis is characterized by extensive fibrosis, microvascular stenosis and autoantibody production. All three characteristics can be accounted for by activation of cells of the immune system. Activation of T cells is antigen-driven and occurs early in the course of the disease, before microscopic evidence of fibrosis. Activated T cells are predominantly of the type 2 T-helper lineage, and produce interleukin-4 and interleukin-13, which induce fibrosis. B cells are also activated early in the course of the disease and, through the production of autoantibodies, cause fibroblasts to adopt a profibrotic phenotype. Macrophages in perivascular infiltrates are activated and produce CC-chemokine ligand 2, transforming growth factor β and platelet derived growth factor, all of which promote fibrosis and fibroproliferation. These new insights have direct impact on the treatment of patients with systemic sclerosis; therapies that target T cells, B cells and their harmful mediators are a logical approach, and preliminary data are promising.
Key Points
-
Immune cells are activated early in the course of systemic sclerosis pathogenesis, even before fibrosis has developed
-
The activation of T cells is antigen-driven, but the antigen (or antigens) is yet unknown
-
Activated T cells are mainly of the type 2 T-helper profile and produce IL-4, which promotes fibrosis and antagonizes the antifibrotic effects of interferon-γ
-
B cells promote fibrosis through the production of autoantibodies
-
Treatments that target immune cells or their harmful soluble mediators have shown promise, and clinical trials are underway
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sakkas LI and Platsoucas CD (2004) Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum 50: 1721–1133
Abraham DJ and Varga J (2005) Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol 26: 587–595
Prescot RJ et al. (1992) Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol 166: 255–263
Kalogerou A et al. (2005) Early T cell activation antigen in the skin from patients with systemic sclerosis. Ann Rheum Dis 64: 1233–1235
Whitfield ML et al. (2003) Systemic and cell-cycle specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci USA 100: 12319–12324
Worda M et al. (2003) In vivo analysis of the apoptosis-inducing effect of anti-endothelial cell antibodies in systemic sclerosis by the chorioallantoic membrane assay. Arthritis Rheum 48: 2605–2614
Baroni SS et al. (2006) Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 354: 2667–2676
Sato S et al. (2004) Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum 50: 1918–1927
Weiner ES et al. (1991) Prognostic significance of anticentromere antibodies and antitopoisomerase I antibodies in Raynaud's disease. Arthritis Rheum 34: 68–77
Kuwana M et al. (1995) T and B cell collaboration is essential for the autoantibody response to DNA topoisomerase I in systemic sclerosis. J Immunol 155: 2703–2714
Black C et al. (1986) Regressive systemic sclerosis. Ann Rheum Dis 45: 384–388
Mavalia C et al. (1997) Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol 151: 1751–1758
Chizollini C et al. (2003) Systemic sclerosis TH2 cells inhibit collagen production in dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Arthritis Rheum 48: 2593–2604
Sakkas LI et al. (1999) Increased levels of alternatively spliced interleukin 4 (IL-4δ2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin Diagn Lab Immunol 6: 660–664
Fujii H et al. (2002) Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis. Clin Exp Immunol 130: 548–556
Matsushita T et al. (2006) Longitudinal analysis of serum cytokine concentrations in systemic sclerosis: association of interleukin 12 elevation with spontaneous regression of skin sclerosis. J Rheumatol 33: 275–284
Kurasawa K et al. (2000) Increased IL-17 production in patients with systemic sclerosis. Arthritis Rheum 43: 2455–2463
Fossiez F et al. (1996) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 183: 2593–2603
Massague J (1990) Transforming growth factor-β family. Annu Rev Cell Biol 6: 597–641
Nabel EG et al. (1993) Direct transfer of transforming growth factor β1 gene into arteries stimulates fibrocellular hyperlpasia. Proc Natl Acad Sci USA 90: 10759–10763
Ihn H et al. (2001) Blockade of endogenous transforming growth factor β signaling prevents upregulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor β receptors. Arthritis Rheum 44: 474–480
Fukasawa C et al. (2003) Increased CD40 expression in skin fibroblasts from patients with systemic sclerosis (SSc): role of CD40–CD154 in the phenotype of SSc fibroblasts. Eur J Immunol 33: 2792–2800
Hasegawa M et al. (2004) Augmented production of transforming growth factor-β by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Arch Dermatol Res 296: 89–93
Duncan MR and Berman B (1991) Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin-6. J Invest Dermatol 97: 686–692
Zhou X et al. (2005) Autoantibodies to fibrillin-1 activate normal human fibroblasts in culture through the TGF-beta pathway to recapitulate the “scleroderma phenotype”. J Immunol 175: 4555–4560
Carvalho D et al. (1996) IgG anti-endothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro: induction of adhesion molecule expression and involvement of endothelium-derived cytokines. J Clin Invest 97: 111–119
Laplante P et al. (2005) Novel fibrogenic pathways are activated in response to endothelial apoptosis: implications in the pathophysiology of systemic sclerosis. J Immunol 174: 5740–5749
Chizzolini C et al. (2002) Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum 46: 1602–1613
Nishijima C et al. (2004) Autoantibody against matrix metalloproteinase-3 in patients with systemic sclerosis. Clin Exp Immunol 138: 357–363
Gu L et al. (2000) Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404: 407–411
Gharaee-Kermani M et al. (1996) Costimulation of fibroblast collagen and transforming growth factor beta 1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 271: 17779–17784
Distler JH et al. (2006) Monocyte chemoattractant protein 1 released from glycosaminoglycans mediates its profibrotic effects in systemic sclerosis via release of interleukin-4 from T cells. Arthritis Rheum 54: 214–225
Motomura Y et al. (2006) Induction of fibrogenic response in mouse colon by overexpression of monocyte chemoattractant protein 1. Gut 55: 662–670
Kitagawa K et al. (2004) Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 165: 237–246
Vancheeswaran R et al. (1994) Localization of endothelin-1 and its binding sites in scleroderma skin. J Rheumatol 21: 1268–1276
Shi-Wen X et al. (2001) Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol 116: 417–425
Xu SW et al. (1998) Endothelin-1 regulation of intercellular adhesion molecule-1 expression in normal and sclerodermal fibroblasts. J Cardiovasc Pharmacol 31 (Suppl 1): S545–S547
Shi-wen X et al. (2000) Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp Cell Res 259: 213–224
Yokoi H et al. (2004) Reduction of connective tissue growth factor by anti-sense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol 15: 1430–1440
Kawaguchi Y et al. (1999) Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest 103: 1253–1260
Giusti B et al. (2005) The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum 52: 3618–3628
Kuwana M et al. (2004) Defective vasculogenesis in systemic sclerosis. Lancet 364: 603–610
Henault J et al. (2006) DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Rheum 54: 963–973
Sakkas LI et al. (2002) Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J Immunol 168: 3649–3659
Arlett C et al. (1998) New prespectives on the etiology of systemic sclerosis. N Engl J Med 338: 1186–1189
Evans PC et al. (1999) Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood 93: 2033–2037
Scaletti C et al. (2002) TH2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum 46: 445–450
Veerarghavan S et al. (2004) Mapping of immunodominant T cell epitopes of the protein topoisomerase I. Ann Rheum Dis 63: 982–987
De Palma R et al. (2006) Peripheral T lymphocytes from patients with early systemic sclerosis co-cultured with autologous fibroblasts undergo an oligoclonal expansion similar to that occurring in the skin. Clin Exp Immunol 144: 169–176
Hasegawa M et al. (2006) B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol 169: 954–966
Ushigayama C et al. (1995) Anti-IL-4 antibody prevents graft-versus host disease in mice after bone marrow transplantation. The IgE allotype is an important marker of graft versus host disease. J Immunol 154: 2687–2696
Charles C et al. (2006) Systemic sclerosis: hypothesis-driven treatment strategies. Lancet 367: 1683–1691
Tashkin DP et al. (2006) Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354: 2655–2666
Straton RJ et al. (2001) Pilot study of anti-thymocyte globulin plus mycophenolate mofetil in recent-onset diffuse scleroderma. Rheumatology 40: 84–88
Swigris JJ et al. (2006) Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-associated interstitial lung disease. Chest 130: 30–36
Liossis N et al. (2006) Mycophenolate mofetil as first line treatment improves clinically evident early scleroderma lung disease. Rheumatology 45: 1005–1008
Scherer HU et al. (2006) Targeting activated T cells: successful use of anti-CD25 monoclonal antibody basiliximab in a patient with systemic sclerosis. Ann Rheum Dis 65: 1245–1247
Levy Y et al. (2004) Intravenous immunoglobulin modulates cutaneous involvement and reduces skin thickness in systemic sclerosis: an open-label trial. Arthritis Rheum 50: 1005–1007
Ratanatharathorn V et al. (2003) Treatment of chronic versus host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant 9: 505–511
Raghu G et al. (2004) A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 350: 125–133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sakkas, L., Chikanza, I. & Platsoucas, C. Mechanisms of Disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Rev Rheumatol 2, 679–685 (2006). https://doi.org/10.1038/ncprheum0346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0346
This article is cited by
-
Hypoxia-Inducible Factor-1α (HIF-1α) as a Biomarker for Changes in Microcirculation in Individuals with Systemic Sclerosis
Dermatology and Therapy (2023)
-
Antigen-specific humoral responses against Helicobacter pylori in patients with systemic sclerosis
Immunologic Research (2020)
-
Attenuation of murine sclerodermatous models by the selective S1P1 receptor modulator cenerimod
Scientific Reports (2019)
-
The Greek (Hellenic) rheumatology over the years: from ancient to modern times
Rheumatology International (2019)
-
Targeting very early systemic sclerosis: a case-based review
Rheumatology International (2019)