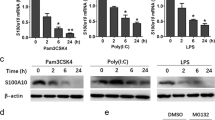
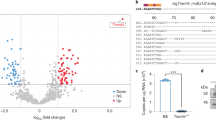
Abstract
Host innate responses to bacterial infections are primarily mediated by neutrophils and monocytes/macrophages1,2. These cells express pattern recognition receptors (PRRs) that bind conserved molecular structures shared by groups of microorganisms3,4. Stimulation of PRR signalling pathways initiates secretion of proinflammatory mediators3,4, which promote the elimination of infectious agents and the induction of tissue repair. Excessive inflammation owing to bacterial infections can lead to tissue damage and septic shock5,6,7,8,9. Here we show that inflammatory responses to microbial products are amplified by a pathway mediated by triggering receptor expressed on myeloid cells (TREM)-1. TREM-1 is an activating receptor expressed at high levels on neutrophils and monocytes that infiltrate human tissues infected with bacteria. Furthermore, it is upregulated on peritoneal neutrophils of patients with microbial sepsis and mice with experimental lipopolysaccaride (LPS)-induced shock. Notably, blockade of TREM-1 protects mice against LPS-induced shock, as well as microbial sepsis caused by live Escherichia coli or caecal ligation and puncture. These results demonstrate a critical function of TREM-1 in acute inflammatory responses to bacteria and implicate TREM-1 as a potential therapeutic target for septic shock.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Medzhitov, R. & Janeway, C. Jr Innate immunity. N. Engl. J. Med. 343, 338–344 (2000).
Hoffmann, J. A., Kafatos, F. C., Janeway, C. A. & Ezekowitz, R. A. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 (1999).
Aderem, A. & Ulevitch, R. J. Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000).
Beutler, B. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr. Opin. Microbiol. 3, 23–28 (2000).
Bone, R. C. The pathogenesis of sepsis. Ann. Intern. Med. 115, 457–469 (1991).
Beutler, B., Milsark, I. W. & Cerami, A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229, 869–871 (1985).
Morrison, D. C. & Ryan, J. L. Endotoxins and disease mechanisms. Annu. Rev. Med. 38, 417–432 (1987).
Tracey, K. J. et al. Shock and tissue injury induced by recombinant human cachectin. Science 234, 470–474 (1986).
Glauser, M. P., Zanetti, G., Baumgartner, J. D. & Cohen, J. Septic shock: pathogenesis. Lancet 338, 732–736 (1991).
Bouchon, A., Dietrich, J. & Colonna, M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 (2000).
Lanier, L. L., Corliss, B. C., Wu, J., Leong, C. & Phillips, J. H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Bernhagen, J. et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365, 756–759 (1993).
Ohlsson, K., Bjork, P., Bergenfeldt, M., Hageman, R. & Thompson, R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348, 550–552 (1990).
Alexander, H. R., Doherty, G. M., Buresh, C. M., Venzon, D. J. & Norton, J. A. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J. Exp. Med. 173, 1029–1032 (1991).
Cella, M. et al. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J. Exp. Med. 185, 1743–1751 (1997).
Echtenacher, B., Falk, W., Mannel, D. N. & Krammer, P. H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145, 3762–3766 (1990).
Echtenacher, B., Mannel, D. N. & Hultner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 (1996).
Malaviya, R., Ikeda, T., Ross, E. & Abraham, S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381, 77–80 (1996).
Rothe, J. et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF- mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364, 798–802 (1993).
Pfeffer, K. et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73, 457–467 (1993).
Peschon, J. J. et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160, 943–952 (1998).
Eskandari, M. K. et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 148, 2724–2730 (1992).
Calandra, T. et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nature Med. 6, 164–170 (2000).
Appelmelk, B. J. et al. Use of mucin and hemoglobin in experimental murine gram-negative bacteremia enhances the immunoprotective action of antibodies reactive with the lipopolysaccharide core region. Antonie Van Leeuwenhoek 52, 537–542 (1986).
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care. Med. 20, 864–874 (1992).
Facchetti, F. et al. Suppurative granulomatous lymphadenitis. Immunohistochemical evidence for a B-cell-associated granuloma. Am. J. Surg. Pathol. 16, 955–961 (1992).
Acknowledgements
We thank R. Breitkreutz and A. Benner for statistical analysis; O. Alebardi and S. Festa for technical assistance in immunohistochemistry; N. Schmitz for advice during mouse experiments; M. Cella, R. Ettinger, F. McBlane, M. Kopf, F. Sinigaglia and R. Torres for reviewing the manuscript; and P. Krammer for discussion. This work was partly supported by a BIOMED-2 grant to F.F. The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche, CH-4002 Basel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouchon, A., Facchetti, F., Weigand, M. et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107 (2001). https://doi.org/10.1038/35074114
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35074114
This article is cited by
-
Triggering receptor expressed on myeloid cells-1 in sepsis, and current insights into clinical studies
Critical Care (2024)
-
Microglia TREM1-mediated neuroinflammation contributes to central sensitization via the NF-κB pathway in a chronic migraine model
The Journal of Headache and Pain (2024)
-
MiR-206 may regulate mitochondrial ROS contribute to the progression of Myocardial infarction via TREM1
BMC Cardiovascular Disorders (2023)
-
TREM-1 triggers necroptosis of macrophages through mTOR-dependent mitochondrial fission during acute lung injury
Journal of Translational Medicine (2023)
-
Macrophage TREM2 as a new player in atherosclerosis
Nature Cardiovascular Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.