Key Points
-
Molecules that crosslink actin filaments into particular architectures are important components of cell structure and movement. Filamins are one of the first of such components recognized and are among the most important.
-
Filamins are extended dimers composed of subunits that contain characteristic β-pleated sheet repeats. Vertebrate filamins have amino-terminal actin-binding domains and self-associate at the carboxyl termini of their subunits.
-
The main human filamin (filamin A) is encoded on the X chromosome. A second filamin gene (filamin B) is encoded on chromosome 3 and a muscle-specific filamin gene (filamin C) is encoded on chromosome 7.
-
So far two filamin genes have been recognized in Drosophila. Dictyostelium amoebae have only one filamin species which is truncated compared with vertebrate and Drosophila filamins.
-
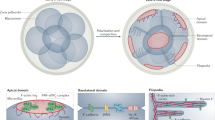
Filamins cause actin filaments to branch with high angles leading efficiently to the formation of actin gels in vitro. The filamins reside at branches between orthogonally intersecting filaments in the peripheral cytoplasm of cells.
-
Filamins also bind over 20 diverse cellular proteins, including membrane receptors and intracellular signalling macromolecules.
-
Cells missing the main filamins have defects in surface stability and locomotion and in some of the functions ascribed to the filamin binding partners. A mutation in the filamin A gene is lethal for males and the cause of periventricular heterotopia in females chimeric for the mutation.
Abstract
Filamins are large actin-binding proteins that stabilize delicate three-dimensional actin webs and link them to cellular membranes. They integrate cellular architectural and signalling functions and are essential for fetal development and cell locomotion. Here, we describe the history, structure and function of this group of proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hartwig, J. & Stossel, T. Isolation and properties of actin, myosin, and a new actin-binding protein in rabbit alveolar macrophages. J. Biol. Chem. 250, 5696?5705 (1975).
Stossel, T. P. & Hartwig, J. H. Interactions of actin, myosin and an actin-binding protein of rabbit alveolar macrophages. Macrophage myosin Mg2+-adenosine triphosphatase requires a cofactor for activation by actin. J. Biol. Chem. 250, 5706? 5712 (1975).
Wang, K., Ash, J. F. & Singer, S. J. Filamin, a new high-molecular weight protein found in smooth muscle and non-muscle cells. Proc. Natl Acad. Sci. USA. 72, 4483?4486 ( 1975).
Shizuta, Y., Shizuta, H., Gallo, M., Davies, P. & Pastan, I. Purification and properties of filamin, an actin-binding protein from chicken gizzard. J. Biol. Chem. 251, 6562?6567 (1976).
Wallach, D., Davies, P. J. A. & Pastan, I. Purification of mammalian filamin. Similarity to high molecular weight actin-binding protein in macrophages, platelets, fibroblasts and other tissues. J. Biol. Chem. 253, 3328 ?3335 (1978).
Bechtel, P. J. Identification of a high molecular weight actin-binding protein in skeletal muscle. J. Biol. Chem. 254, 1755? 1758 (1979).
Condeelis, J., Salisbury, J. & Fujiwara, K. A new protein that gels F actin in the cell cortex of Dictyostelium discoideum. Nature 292, 161?163 (1981).
Condeelis, J., Geosits, J. & Vahey, M. Isolation of a new actin binding protein from Dictyostelium . Cell Motility 2, 273? 285 (1982).
Vargas, M., Sansonetti, P. & Guillén, N. Identification and cellular localization of the actin-binding protein ABP-120 from Entamoeba histolytica. Mol. Microbiol. 22, 849?857 ( 1996).
Noegel, A., Rapp, S., Lottspeich, F., Schleicher, M. & Stewart, M. The Dictyostelium gelation factor shares a putative actin binding site with α-actinins and dystrophin and also has a rod domain containing six 100-residue motifs that appear to have a cross-beta conformation. J. Cell Biol. 109, 607? 618 (1989).
Li, M. -G. et al. Filamin is required for ring canal assembly and actin organization during Drosophila oogenesis. J. Cell Biol. 146 , 1061?1073 (1999).
Sokol, N. & Cooley, L. Drosophila filamin encoded by the cheerio locus is a component of ovarian ring canals. Curr. Biol. 9, 1221?1230 (1999).Introduction to Drosophila oocyte development and overview of filamins in Drosophila.
Weihing, R. R. The filamins: properties and functions. Can. J. Biochem. Cell Biol. 63, 397?413 ( 1985).
Hartwig, J. in Protein Profile (ed. Sheterline, P.) 711?778 (Academic, London, 1994).
Lebart, M. -C. et al. Characterization of the actin binding site on smooth muscle filamin. J. Biol. Chem. 269, 4279? 4284 (1994).
Gorlin, J. et al. Human endothelial actin-binding protein (ABP, nonmuscle filamin): a molecular leaf spring. J. Cell Biol. 111, 1089?1105 (1990). This paper describes the cloning and structure of FLNa.
Taylor, D. & Condeelis, J. Cytoplasmic structure and contractility in amoeboid cells. Int. Rev. Cytol. 56, 57?144 (1979).
Brotschi, E. A., Hartwig, J. H. & Stossel, T. P. The gelation of actin by actin-binding protein. J. Biol. Chem. 253, 8988?8993 (1978).
Bennett, J., Zaner, K. & Stossel, T. Isolation and some properties of macrophage α-actinin. Evidence that it is not an actin gelling protein. Biochemistry 23, 5081?5086 ( 1984).
Ito, T., Suzuki, A. & Stossel, T. Regulation of water flow by actin-binding protein-induced actin gelation. Biophys. J. 61, 1301? 1305 (1992).
Goldmann, W. & Isenberg, G. Analysis of filamin and α-actinin binding to actin by the stopped flow method. FEBS Lett. 336, 408?410 (1993).
Matsudaira, P. Actin crosslinking proteins at the leading edge. Semin. Cell Biol. 5, 165?174 ( 1994).
Honda, M., Takiguchi, K., Ishikawa, S. & Hotani, H. Morphogenesis of liposomes encapsulating actin depends on the type of actin-crosslinking . J. Mol. Biol. 287, 293? 300 (1999).
Stossel, T. & Hartwig, J. Interactions of actin, myosin and an actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J. Cell Biol. 68, 602?614 (1976).
Hartwig, J. H., Tyler, J. & Stossel, T. P. Actin-binding protein promotes the bipolar and perpendicular branching of actin filaments. J. Cell Biol. 87, 841?848 (1980).
Niederman, R., Amrein, P. & Hartwig, J. H. The three dimensional structure of actin filaments in solution and an actin gel made with actin-binding protein. J. Cell Biol. 96, 1400?1413 (1983).
Wolosowick, J. & Condeelis, J. Fine structure of gels prepared from an actin-binding protein: comparison to cytoplasmic extracts and cortical cytoplasm in ameboid cells of Dictyostelium discoideum . J. Cell Biochem. 30, 227? 243 (1986).
Weihing, R. R. Actin-binding and dimerization domains of HeLa cell filamin. Biochemistry 27, 1865?1869 ( 1988).
Bresnick, A., Warren, V. & Condeelis, J. Identification of a short sequence essential for actin binding by Dictyostelium ABP-120. J. Biol. Chem. 265, 9236?9240 (1990).
Bresnick, A., Janmey, P. & Condeelis, J. Evidence that a 27-residue sequence is the actin-binding site of ABP-120. J. Biol. Chem. 266, 12989 ?12993 (1991).
Hartwig, J. H. & Stossel, T. P. The structure of actin-binding protein molecules in solution and interacting with actin filaments. J. Mol. Biol. 145, 563? 581 (1981).
Schliwa, M. & Van Blerkom, J. Structural interactions of cytoskeletal components. J. Cell Biol. 90, 225? 235 (1981).
Hartwig, J. H. & Shevlin, P. The architecture of actin filaments and the ultrastructural location of actin-binding protein in the periphery of lung macrophages. J. Cell Biol. 103, 1007?1020 (1986).
Hartwig, J. H., Chambers, K. A. & Stossel, T. P. Assocation of gelsolin with actin filaments and cell membranes of macrophages and platelets. J. Cell Biol. 108, 467?480 (1989).
Hartwig, J. Mechanisms of actin rearrangements mediating platelet activation. J. Cell Biol. 118, 1421?1442 (1992).
Bailly, M. et al. Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J. Cell Biol. 145, 331 ?345 (1999).
Svitkina, T. & Borisy, G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009?1026 (1999).
Langanger, G. et al. Ultrastructural localization of α-actinin and filamin in cultured cells with the immunogold staining (IGS) method. J. Cell Biol. 99, 1324?1334 (1984).
Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404 , 1007?1011 (2000).
Pantaloni, D., Boujemaa, R., Didry, D., Gounon, P. & Carlier, M. -F. The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins. Nature Cell Biol. 2, 385?391 ( 2000).
Mullins, R., Heuser, M. & Pollard, T. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181?6186 (1998).
Mullins, R., Kelleher, J., Xu, J. & Pollard, T. Arp2/3 complex from Acanthamoeba binds profilin and crosslinks actin filaments. Mol. Biol. Cell 9, 841?852 (1998).
Janmey, P. A., Hvidt, S., Lamb, J. & Stossel, T. P. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature 345, 89?92 ( 1990).This paper describes the rheological background of filamin?actin gels.
Janssen, K.-P. et al. Viscoelastic properties of F-actin solutions in the presence of normal and mutated actin-binding proteins. Arch. Biochem. Biophys. 325, 183?189 ( 1996).
Bishop, A. & Hall, A. Rho GTPases and their effector proteins . Biochem. J. 348, 241? 255 (2000).
Marti, A. et al. ABP-280 binds the SAPK activator SEK-1 and is required for TNFα activation of SAPK in melanoma cells. J. Biol. Chem. 272, 2620?2628 (1997).
Ohta, Y., Suzuki, N., Nakamura, S., Hartwig, J. & Stossel, T. The small GTPase RalA targets filamin to induce filopodia . Proc. Natl Acad. Sci. USA 96, 2122? 2128 (1999).
Bellanger, J. -M. et al. The Rac1- and RhoG-activating domain of the bifunctional guanine nucleotide exchange factor Trio targets filamin to remodel cytoskeletal actin . Nature Cell Biol. 2, 888? 892 (2000).
Fisher, P. et al. Photosensory and thermosensory responses in Dictyostelium slugs are specifically impaired by absence of the F-actin crosslinking gelation factor (ABP-120). Curr. Biol. 7, 889?892 (1997).
Glogauer, M. et al. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 273, 1689 ?1698 (1998).
Wallach, D., Davies, P. J. A. & Pastan, I. Cyclic AMP-dependent phosphorylation of filamin in mammalian smooth muscle. J. Biol. Chem. 253, 4739? 4745 (1978).
Chen, M. & Stracher, A. In situ phosphorylation of platelet actin-binding protein by cAMP-dependent protein kinase stabilizes it against proteolysis by calpain. J. Biol. Chem. 264 , 14282?14289 (1989).
Kawamoto, S. & Hidaka, H. Ca2+-activated phospholipid-dependent protein kinase catalyses the phosphorylation of actin-binding proteins. Biochem. Biophys. Res. Commun. 118, 736? 742 (1984).
Jay, D., García, E., Lara, J., Medina, M. & de la Cruz Ibarra, M. Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch. Biochem. Biophys. 377, 80?84 (2000).
Ohta, Y. & Hartwig, J. Phosphorylation of actin-binding protein 280 by growth factors is mediated by p90 ribosomal protein S6 kinase . J. Biol. Chem. 271, 11858? 11864 (1996).
Ohta, Y. & Hartwig, J. Actin filament crosslinking by chicken gizzard filamin is regulated by phosphorylation in vitro. Biochemistry. 34, 6745?6754 (1995).
Cox, D. et al. Targeted disruption of the ABP-120 gene leads to cells with altered motility. J. Cell Biol. 116, 943? 955 (1992).
Cox, D., Wessels, D., Soll, D., Hartwig, J. & Condeelis, J. Re-expression of ABP-120 rescues cytoskeletal, motility, and phagocytosis defects of ABP-120-Dictyostelium mutants. Mol. Biol. Cell 7, 803?823 (1996).
Ponte, E., Rivero, F., Fechheimer, M., Noegel, A. & Bozzaro, S. Severe developmental defects in Dictyostelium null mutants for actin-binding proteins. Mech. Dev. 91, 153?161 ( 2000).
Cox, D., Ridsdale, J., Condeelis, J. & Hartwig, J. Genetic deletion of ABP-120 alters the three-dimensional organization of actin filaments in Dictyostelium pseudopods. J. Cell Biol. 128, 819?835 (1995). Description of filamin-deficient Dictyostelium amoebae.
Rivero, F. et al. The role of the cortical cytoskeleton: F-actin crosslinking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J. Cell Sci. 109, 2679?2691 ( 1996).
Rivero, F., Furukawa, R., Fechheimer, M. & Noegel, A. Three actin crosslinking proteins, the 34 kDa actin-bundling protein, α-actinin and gelation factor (ABP-120), have both unique and redundant roles in the growth and development of Dictyostelium. J. Cell Sci. 112, 2737?2751 (1999).
Cunningham, C. et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255, 325? 327 (1992).Description of filamin-deficient melanoma cells and their rescue.
Dai, J. & Sheetz, M. Membrane tether formation from blebbing cells. Biophys. J. 77, 3363? 3370 (1999).
Cunningham, C. Actin polymerization and intracellular solvent flow in cell surface blebbing . J. Cell Biol. 129, 1589? 1599 (1995).
Fox, J. et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 21, 1315?1325 (1998). Description of the first human filamin mutation, periventricular heterotopia.
Robinson, D., Smith-Leiker, T., Sokol, N., Hudson, A. & Cooley, L. Formation of the Drosophila ovarian ring canal inner rim depends on cheerio. Genetics 145, 1063?1072 (1997).
Ezzell, R. M., Kenney, D. M., Egan, S., Stossel, T. P. & Hartwig, J. H. Localization of the domain of actin-binding protein that binds to membrane glycoprotein Ib and actin in human platelets. J. Biol. Chem. 263, 13303?13309 (1988).
Tyler, J. M., Anderson, J. M. & Branton, D. Structural comparison of several actin-binding molecules . J. Cell Biol. 85, 489? 495 (1980).
Fucini, P., Renner, C., Heberhold, C., Noegel, A. & Holak, T. The repeating segments of the F-actin crosslinking gelation factor (ABP-120) have an immunoglobulin-like fold. Nature Struct. Biol. 4, 223?230 (1997).
Fucini, P. et al. Molecular architecture of the rod domain of the Dictyostelium gelation factor (ABP-120). J. Mol. Biol. 291, 1017?1023 (1999).
McCoy, A., Fucini, P., Noegel, A. & Stewart, M. Structural basis for dimerization of the Dictyostelium gelation factor (ABP120) rod . Nature Struct. Biol. 9, 836? 841 (1999).This paper presents the atomic structure of a Dictyostelium filamin self-association domain.
Hartwig, J. & DeSisto, M. The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. J. Cell Biol. 112, 407 ?425 (1991).
Gorlin, J. et al. Actin-binding protein (ABP-280) filamin gene (FLN) maps telomeric to the color vision locus (R/CGP) and centromeric to G6PD in Xq28. Genomics 17, 496?498 ( 1993).
Maestrini, E. et al. Mapping of two genes encoding isoforms of the actin-binding protein ABP-280, a dystrophin like protein, to Xq28 and to chromosome 7. Hum. Mol. Genet. 2, 761?766 (1993).
Zhang, W., Han, S., McKeel, D., Goate, A. & Wu, J. Interaction of presenilins with the filamin family of actin-binding proteins . J. Neurosci. 18, 914? 922 (1998).
Takafuta, T., Wu, G., Murphy, G. & Shapiro, S. Human β-filamin is a new protein that interacts with the cytoplasmic tail of glycoprotein Ibα. J. Biol. Chem. 273, 17531? 17538 (1998).
Xu, W. -F, Xie, Z. -W, Chung, D. & Davie, E. A novel human actin-binding protein homologue that binds to platelet glycoprotein Ibα. Blood 92, 1268?1276 ( 1998).
Bröcker, F. et al. Assignment of human filamin gene FLNB to human chromosome band 3p14. 3 and identification of YACs containing the complete FLNB transcribed region. Cytogenet. Cell Genet. 85, 267? 268 (1999).
Chakarova, C. et al. Genomic structure and fine mapping of the two human filamin gene paralogues FLNB and FLNC and comparative analysis of the filamin gene family. Hum. Genet. 107, 597? 611 (2000).
Barry, C. P., Xie, J., Lemmon, V. & Young, A. P. Molecular characterization of a multipromoter gene encoding a chicken filamin protein. J. Biol. Chem. 268, 25577?25586 (1993).
Xie, Z., Wu, W., Davie, E. & Chung, D. Molecular cloning of human ABPL, an actin-binding protein homologue. Biochem. Biophys. Res. Commun. 251, 914?919 (1998).
van der Ven, P., Obermann, W., Lemke, B., Gautel, M., Weber, K. & Fürst, D. Characterization of muscle filamin isoforms suggests a possible role of γ-filamin/ABP-L in sarcomeric Z-disc formation. Cell Motil. Cytoskeleton 45, 149?162 (2000).
van der Ven, P. et al. Indications for a novel muscular dystrophy pathway: γ-filamin, the muscle-specific filamin isoform, interacts with myotilin. J. Cell Biol. 151, 235?247 (2000).
Meyer, S. et al. Identification of the region in actin-binding protein that binds to the cytoplasmic domain of glycoprotein Ib. J. Biol. Chem. 272, 2914?2919 (1997).
Calderwood, D., Shattil, S. & Ginsberg, M. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 275, 22607?22610 (2000).
Ott, I., Fischer, E., Miyagi, Y., Mueller, B. & Ruf, W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J. Cell Biol. 140, 1241?1253 ( 1998).
Ohta, Y., Stossel, T. & Hartwig, J. Ligand-sensitive binding of actin-binding protein (ABP) to immunoglobulin G Fc receptor I(FcγR1, CD64). Cell. 67, 275?282 (1991).
Liu, G. et al. Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway. J. Cell Biol. 139, 1719?1733 (1997).Good example of the identification and functional workup of a filamin?partner relationship.
Thompson, T. et al. Filamin 2 (FLN2): a muscle-specific sarcoglycan interacting protein. J. Cell Biol. 148, 115? 126 (2000).
Stahlhut, M. & van Deurs, B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell 11, 325?337 (2000).
Schwartzman, A. et al. Endogenous presenilin 1 redistributes to the surface of lamellipodia upon adhesion of Jurkat cells to a collagen matrix. Proc. Natl Acad. Sci. USA 96, 7932?7937 (1999).
Guo, Y., Zhang, S., Sokol, N., Cooley, L. & Boulianne, G. Physical and genetic interaction of filamin with presenilin in Drosophila. J. Cell Sci. 113, 3499?3508 (2000).
Li, M., Bermak, C., Wang, Z. & Zhou, Q. Modulation of dopamine D2 receptor signaling by actin-binding protein (ABP-280). Mol. Pharmacol. 57, 446?452 (2000).
Browne, K., Johnstone, R., Jans, D. & Trapani, J. Filamin (280-kDa actin-binding protein) is a caspase substrate and is also cleaved directly by the cytotoxic T lymphocyte protease granzyme B during apoptosis. J. Biol. Chem. 275, 39262?39266 (2000).
Edwards, D., Towb, P. & Wasserman, S. An activity-dependent network of interactions links the Rel protein Dorsal with its cytoplasmic regulators. Development 124, 3855?3864 ( 1997).
Leonardi, A., Ellinger-Ziegelbauer, H., Franzoso, G., Brown, K. & Siebenlist, U. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis receptor-associated factor 2. J. Biol. Chem. 275, 271? 278 (2000).
Ozanne, D., Brady, M., Gaughan, L., Cook, S., Neal, D. & Robson, C. Androgen receptor nuclear translocation is faciliated by the F-actin crosslinking protein filamin . Mol. Endocrinol. 14, 1618? 1626 (2000).
Krief, S. et al. Identification and characterization of cvHsp. A novel human small stress protein selectively expressed in cardiovascular and insulin-sensitive tissues. J. Biol. Chem. 274, 36592? 36600 (1999).
Petrecca, K., Miller, D. & Shrier, A. Localization and enhanced current density of the Kv4. 2 potassium channel by interaction with the actin-binding protein filamin . J. Neurosci. 20, 8736? 8744 (2000).
Acknowledgements
Some of the research reported in this review was supported by the NIH grants and by the Edwin S Webster Foundation (to T.P.S and J.H.H). We appreciate comments on this paper by Kuan Wang, NIAMS, NIH, who with S. John Singer was the first to purify avian gizzard filamin.
Author information
Authors and Affiliations
Additional information
Movie. Morphology of FLNa-repleted cells (left) derived from FLNa-null M2 cells (right). Filamin-expressing cells ruffle and begin to translocate, whereas M2 cells remain stationary and bleb.
Supplementary information
Related links
Related links
DATABASE LINKS
Ca2+/calmodulin-dependent protein kinase II
human periventricular heterotopia
FURTHER INFORMATION
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- PHAGOCYTOSIS
-
Actin-dependent process, by which cells engulf external particulate material by extension and fusion of pseudopods.
- OSMOTIC FLUID FLOW
-
The movement of fluid across semi-permeable membranes from lesser to greater solute concentrations.
- HYDROSTATIC FLUID FLOW
-
The movement of fluid under mechanical pressure in the direction of least resistance.
- MACROPHAGE
-
Any cell of the mononuclear phagocyte system that is characterized by its ability to phagocytose foreign particulate and colloidal material.
- FIBROBLAST
-
Common cell type found in connective tissue in many parts of the body, which secretes an extracellular matrix rich in collagen and other macromolecules.
- PARALOGUE
-
Gene products on opposite branches of a duplicated gene family. Orthologues are on the same branch (for example, FLNa, FLNb and FLNc). Paralogues and orthologues are homologues.
- OVARIAN RING CANAL
-
In Drosophila, a single oocyte develops in egg chambers containing 15 nurse cells connected by intercellular bridges ? the ring canals.
- GEL
-
A liquid becomes a gel when a 'giant molecule' occupies the entire liquid.
- POINTED END
-
Defined by arrowhead appearance of myosin head fragments bound to the actin filaments.
- LEADING EDGE
-
The thin margin of a lamellipodium spanning the area of the cell from the plasma membrane to about 1 μm back into the lamellipodium.
- PLATELETS
-
The smallest blood cells, which are important in haemostasis and blood coagulation.
- MELANOMA
-
Cancer derived from melanocytes, the cells that synthesize melanin pigments.
- RHO FAMILY GTPASES
-
Ras-related GTPases involved in controlling the polymerization of actin.
- DICTYOSTELIUM SLUGS
-
Aggregated form of Dictyostelium amoebae.
- CALPAIN
-
Calcium-dependent cysteine proteases involved in signal transduction in a variety of cellular processes.
- LAMELLA
-
Flat, sheet-like protrusions at the edge of the cell. A fan-shaped lamella is a prominent feature identifying the leading edge of a cell undergoing locomotion on a flat surface. Actin networks are the principal structures within these lamellae.
- EPILEPTIC SEIZURE
-
Commonly known as fits, seizures are the result of uncontrolled bursts of neuronal activation in the brain that usually lead to repetitive motor activity, such as shaking of extremities. Epilepsy is the term applied to recurrent seizures. Anatomical malformations, tumours, scars, infection, inflammation, haemorrhage and metabolic derangements can cause seizures.
- LATERAL VENTRICLES
-
Cavernous structures in the middle of the cerebrum that contain cerebrospinal fluid.
Rights and permissions
About this article
Cite this article
Stossel, T., Condeelis, J., Cooley, L. et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2, 138–145 (2001). https://doi.org/10.1038/35052082
Issue Date:
DOI: https://doi.org/10.1038/35052082
This article is cited by
-
Role of the Ror family receptors in Wnt5a signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Filamin A organizes γ‑aminobutyric acid type B receptors at the plasma membrane
Nature Communications (2023)
-
Filamin A cooperates with the androgen receptor in preventing skeletal muscle senescence
Cell Death Discovery (2023)
-
Direct and Indirect Effects of Filamin A on Tau Pathology in Neuronal Cells
Molecular Neurobiology (2023)
-
Intervertebral disc degeneration is rescued by TGFβ/BMP signaling modulation in an ex vivo filamin B mouse model
Bone Research (2022)