Abstract
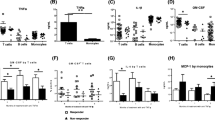
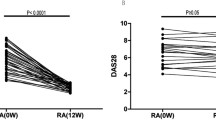
To investigate the mechanism of the long-lasting efficacy of chimeric monoclonal anti-TNFα antibody (cA2) therapy for rheumatoid arthritis (RA), eight patients with refractory RA were treated with a single infusion of cA2 and the changes in circulating cytokines (IL-1, IL-6, TNFα, and IL-10), soluble cytokine receptors (TNF-RI, RII, and sIL-6R) and peripheral white blood cell (WBC) subset counts were followed up long-term (12 weeks) after cA2 therapy in them. Significant clinical responses (>20% improvement according to Paulus' criteria) were observed just after cA2 infusion and lasted more than 4 weeks in all patients, as reported elsewhere. Moreover, five of the eight patients showed prolonged clinical responses (>12 weeks). The elevated serum IL-6 and sTNF-RI (or RII) levels before treatment rapidly decreased after treatment. The serum IL-10 levels also significantly elevated before treatment. The elevations of serum IL-10 levels were augmented after treatment and stayed higher than the baseline in four patients with prolonged clinical responses. No significant TNFα, IL-1α and -β, or sIL-6R were detected in the sera of the patients before treatment and during the whole study period. On the other hand, peripheral lymphocytes as well as total WBC and neutrophils increased for 4 weeks after treatment. However, thereafter, only the lymphocyte count decreased gradually and stayed below the baseline long-term (12 weeks). FACS analysis revealed the predominance of T lymphocytes in the decrease in lymphocyte counts. These results suggest that the augmentation of IL-10 production and the decrease in T cells might partly contribute to the long-lasting efficacy of cA2 treatment in RA.
Similar content being viewed by others
REFERENCES
Panayi GS, Lanchbury JS, Kingsley GH: The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arth Rheum 35:729–735, 1992
Mima T, Saeki Y, Ohshima S, et al.: Transfer of rheumatoid arthritis into severe combined immunodeficient mice; The pathogenic implications of T cell populations oligoclonally expanding in the rheumatoid joints. J Clin Invest 96:1746–1758, 1995
Feldmann M, Brennan FM, Maini RN: Role of cytokines in autoimmune disease. In Clinical Applications of Cytokines; Role in Pathogenesis, Diagnosis, and Therapy, A Gearing, J Rossio, J Oppenheim (eds). Oxford, Oxford University Press, 1993
Arend WP, Drayer JM: Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arth Rheum 38:151–160, 1995
Miyasaka N, Sato K, Goto M, et al.: Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients possible involvement in joint destruction. Arth Rheum 31:480–486, 1988
Duff GW, Dickens E, Wood N, et al.: Immunoassay, bioassay and in situ hybridization of monokines in human arthritis. Prog Leucocyte Bio 8:387, 1988
Buchan G, Barrett K, Turner M, et al.: Interleukin-1 and tumor necrosis factor mRNA expression in rheumatoid arthritis; Prolonged production of IL-1α. Clin Exp Immunol 73:445–449, 1988
Chu CQ, Field M, Feldmann M, et al.: Localization of tumor necrosis factor α in synovial tissues and at the cartilage pannus junction in patients with rheumatoid arthritis. Arth Rheum 34:1125–1132, 1991
Field M, Chu CQ, Feldmann M, et al.: IL-6 localization in the synovial membrane in rheumatoid arthritis. Rheumatol Int 11:45–50, 1991
Firestein GS, Alvaro Grcia JM, Maki R: Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol 144:3347–3353, 1990
Tan PL, Farmiloe S, Yeoman S, et al.: Expression of the interleukin 6 gene in rheumatoid synovial fibroblasts. J Rheumatol 17:1608–1612, 1990
Wood NC, Symons JA, Dickens E, et al.: In situ hybridization of IL-6 in rheumatoid arthritis. 87:183–189, 1992
Lebsack ME, Paul CC, Matindale JJ, et al.: A dose and regimenranging study of IL-1 receptor antagonist in patients with rheumatoid arthritis. Arth Rheum 36:s39, 1993
Drevlow B, Capezio J, Lovis R, et al.: Phase I study of recombinant human interleukin-1 receptor (RHU IL-R) administered intraarticularly in active rheumatoid arthritis. Arth Rheum 36:s39, 1993
Moreland LW, Baumgartner SW, Schiff MH, et al.: Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 337(3):141–147, 1997
Wendling D, Racadot E, Wijdenes J: Treatment of severe rheumatoid arthritis by anti-interleukin 6 monoclonal antibody. J Rheumatol 20:259–262, 1993
Elliott MJ, Maini RN, Feldmann M, et al.: Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arth Rheum 36:1681–1690, 1993
Elliott MJ, Maini RN, Feldmann M, et al.: Randomised double blind comparison of chimeric monoclonal antibody to tumor necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 344:1105–1110, 1994
Elliott MJ, Maini RN, Feldmann M, et al.: Repeated therapy with monoclonal antibody to tumor necrosis factor α (cA2) in patients with rheumatoid arthritis. Lancet 344:1125–1128, 1994
Rankin ECC, Choy EHS, Kassimos D, et al.: The therapeutic effects of an engineered human anti-tumor necrosis factor alpha antibody (CD571) in rheumatoid arthritis. Br J Rheumatol 34:334–342, 1995
Arnett FC, Edworthy SM, Bloch DA, et al.: The American Rheumatism Association 1987 revised criteria for classification of rheumatoid arthritis. Arth Rheum 31:315–324, 1988
Steinbrocker O, Traeger CH, Batterman RC: Therapeutic criteria in rheumatoid arthritis. JAMA 140:659–662, 1949
Knight DM, Trinh H, Le J, et al.: Construction and initial characterization of a mouse-human anti-TNFα antibody. Mol Immunol 30:1443–1453, 1993
Paulus HE, Egger MJ, Ward JR, Williams HJ: The Cooperative Systematic Studies of Rheumatic Disease Group. Analysis of improved in individual rheumatoid arthritis patients treated with disease modifying anti-rheumatic drugs, based on the findings in patients treated with placebo. Arth Rheum 33:477–484, 1990
Kobayashi Y, Sawada JI, Osawa T: Activation of membrane phospholipase A by guinea pig lymphotoxin (GLT). J Immunol 122:791–794, 1979
Ogata A, Tagoh H, Lee T, et al.: A new highly sensitive immunoassay for cytokins by dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA). J Immunol Methods 148:15–22, 1992
Abrams JS, Roncarolo MG, Yssel H, et al.: Strategies of anticytokine monoclonal antibody development; Immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev 127:5–24, 1992
Paleolog EM, Hunt M, Elliott MJ, Feldmann M, Maini RN, Woody JN: Deactivation of vascular endothelium by monoclonal antitumor necrosis factor α antibody in rheumatoid arthritis. Arth Rheum 39:1082–1091, 1996
Swaak AJ, van Rooyen A, Nieuwenhuis E, et al.: Interleukin 6 (IL-6) in synovial fluid and serum of patients with rheumatic disease. Scand J Rheumatol 17:469–474, 1988
Houssiau FA, Devogelaer JP, van Damme J, et al.: Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritis. Arth Rheum 31:784–788, 1988
van Damme J, Opdenakker G, Simpson RJ, et al.: Identification of the human 26-kD protein, interferon b2, as a B cell hybridoma/plasmacytoma growth factor induced by interleukin-1 and tumor necrosis factor. J Exp Med 165:914–919, 1987
Dinarello CA, Cannon JG, Wolff SM, et al.: Tumor necrosis factor (cachectin) is an endogenous pyrogen and induced production of interleukin-1. J Exp Med 163:1433–1450, 1986
Brenann FM, Chantry D, Jakson A, Maini RN, Feldmann M: Inhibitory effects of TNFα antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet 2:244–247, 1989
Butler D, Maini RN, Feldmann M, Brenann FM: Blockade of TNFα with chimeric anti-TNFα monoclonal antibody, cA2 reduces (IL-6 and IL-8) release in RA MNC cultures. A comparison with IL-1ra. Eur Cytokine Netwk 6:225–230, 1995
Haworth C, Brenann FM, Chantry D, Turner M, Maini RN, Feldmann M: Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest 83:876–882, 1991
Feldmann M, Brennan FM, Maini RN: Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 14:397–440, 1996
Cush TJ, Splawski JB, Thomas R, et al.: Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arth Rheum 38:96–104, 1995
Isomaki P, Luukkainen R, Saario R, Toivanen P, Punnonen J: Interleukin-10 functions as an anti-inflammatory cytokine in rheumatoid synovium. Arth Rheum 39:386–395, 1996
Mosmann TR: Properties and functions of interleukin-10. Adv Immunol 56:1–26, 1994
De Waal Malefyt R, Abrams J, Bennett B, Fidgor CG, de Vries JE: Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220, 1991
De Waal Malefyt R, Johnson K, Kastelein R, et al.: Interleukin-10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 174:915–924, 1991
De Waal Malefyt R, Yssel H, de Vries JE: Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. J Immunol 150:4754–4765, 1993
Liblan RS, Singer SM, McDevitt HO: Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune disease. Immunol Today 16:34–38, 1995
Cope AP, Londei M, Chu R, et al.: Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; Reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest 94:749–760, 1994
McCall JL, Yun K, Funamoto S, et al.: In vivo immunohistochemical identification of tumor necrosis factor/cachectin in lymphoid tissue. Am J Pathol 135:421–425, 1989
de Kossodo S, Gran GE, Daneva T, et al.: Tumor necrosis factor α is involved in mouse growth and lymphoid tissue development. J Exp Med 176:1259–1264, 1992
Vella AT, McCormack JE, Linsley PS, et al.: Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity 2:261–270, 1995
Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J: Chimeric anti-TNFα monoclonal antibody cA2 binds recombinant transmembrane TNFα and activates immune effector functions. Cytokine 7:251–259, 1995
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohshima, S., Saeki, Y., Mima, T. et al. Long-Term Follow-Up of the Changes in Circulating Cytokines, Soluble Cytokine Receptors, and White Blood Cell Subset Counts in Patients with Rheumatoid Arthritis (RA) After Monoclonal Anti-TNFα Antibody Therapy. J Clin Immunol 19, 305–313 (1999). https://doi.org/10.1023/A:1020543625282
Issue Date:
DOI: https://doi.org/10.1023/A:1020543625282