Abstract
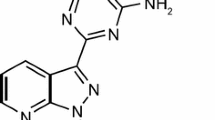
Riociguat (Adempas®), an oral first-in-class soluble guanylate cyclase (sGC) stimulator, is under global development by Bayer Healthcare Pharmaceuticals Inc. for the treatment of adult patients with inoperable or chronic/persistent chronic thromboembolic pulmonary hypertension (CTEPH) and for the treatment of adult patients with pulmonary arterial hypertension (PAH). The drug directly stimulates sGC in a nitric oxide independent manner, thereby increasing the sensitivity of sGC to nitric oxide, leading to increased cyclic guanosine monophosphate generation (a key signalling molecule involved in regulating vascular tone, proliferation, fibrosis and inflammation). Riociguat is the world’s first approved pharmacotherapy for CTEPH, with its first global approval in this indication occurring in Canada. It has subsequently been approved in the USA for the treatment of patients with CTEPH and also received its first global approval in patients with PAH in the USA. It is undergoing regulatory review for these indications in Europe and for use in patients with CTEPH in Japan. This article summarizes the milestones in the development of riociguat, leading to its first global approvals in patients with CTEPH and PAH.
Similar content being viewed by others
References
Agarwal R, Gomberg-Maitland M. Current therapeutics and practical management strategies for pulmonary arterial hypertension. Am Heart J. 2011;162(2):201–13.
Archer SL. Riociguat for pulmonary hypertension: a glass half full. N Engl J Med. 2013;369(4):386–8.
Delcroix M. Chronic post-embolic pulmonary hypertension: a new target for medical therapies? Eur Respir Rev. 2013;22(129):258–64.
Gheorghiade M, Marti CN, Sabbah HN, et al. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Fail Rev. 2013;18(2):123–34.
Ghofrani HA, Grimminger F. Soluble guanylate cyclase stimulation: an emerging option in pulmonary hypertension therapy. Eur Respir Rev. 2009;18(111):35–41.
Boerrigter G, Burnett JC. Soluble guanylate cyclase: not a dull enzyme. Circulation. 2009;119(21):2752–4.
Bayer Inc. Product monograph: ADEMPAS® riociguat (film-coated) tablet; 2013. http://www.bayer.ca/?q=en/node/62 (Accessed 7 Oct 2013).
Bayer Inc. Bayer receives approval for Adempas® as first drug to treat rare heart and lung disease; 2013. http://www.bayer.com (Accessed 7 Oct 2013).
US Food and Drug Administration. FDA approves Adempas to treat pulmonary hypertension; 2013. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm370866.htm (Accessed 29 Oct 2013).
Bayer HealthCare Pharmaceuticals Inc. Adempas (riociguat) tablets, for oral use: US prescribing information; 2013. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf (Accessed 29 Oct 2013).
Bayer HealthCare. Bayer’s riociguat for patients with chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension submitted for regulatory approval in the U.S. and EU; 2013. http://www.bayer.com (Accessed 7 Oct 2013).
Bayer Inc. Bayer submits investigational drug riociguat for patients with chronic thromboembolic pulmonary hypertension for regulatory approval in Japan; 2013. http://www.bayerpharma.com (Accessed 7 Oct 2013).
Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–29.
Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–40.
Bayer. BAY63-2521: long-term extension study in patients with chronic thromboembolic pulmonary hypertension (CHEST-2) [ClinicalTrials.gov identifier NCT009110429]. US National Institutes of Health, ClinicalTrials.gov; 2013. http://www.clinicaltrials.gov (Accessed 8 Oct 2013).
Bayer. BAY63-2521: long-term extension study in patients with pulmonary arterial hypertension (PATENT-2) [ClinicalTrials.gov identifier NCT00863681]. US National Institutes of Health, ClinicalTrials.gov; 2013. http://www.clinicaltrials.gov (Accessed 10 Oct 2013).
Bayer Healthcare Pharmaceuticals Inc. Briefing document for Cardiovascular and Renal Drugs Advisory Committee: riociguat (BAY 63-2521); 2013. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm363543.pdf (Accessed 8 Oct 2013).
Mittendorf J, Weigand S, Alonso-Alija C, et al. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4(5):853–65.
Frey R, Muck W, Unger S, et al. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol. 2008;48:926–34.
Becker EM, Stasch J-P, Bechem M, et al. Effects of different pulmonary vasodilators on arterial saturation in a model of pulmonary hypertension. PLoS ONE. 2013;8(8):e73502.
Lang M, Kojonazarov B, Tian X, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS ONE. 2012;7(8):e43433.
Evgenov OV, Zou L, Zhang M, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase attenuates pulmonary fibrosis [abstract]. BMC Pharmacol. 2011;11(Suppl 1):O9.
Becker EM, Stasch JP, Bechem M, et al. Comparison of different vasodilators, endothelin antagonist, PDE5 inhibitior and sGC stimulators, in an animal model of secondary pulmonary hypertension: effects on “desaturation” [abstract]. BMC Pharmacol. 2011;11(Suppl 1):P5.
Kojonazarov B, Lang M, Weissmann N, et al. Effects of riociguat on pulmonary vascular remodeling in severe experimental pulmonary hypertension [abstract]. Am J Respir Crit Care Med. 2011;183(Suppl):A2516.
Pichl A, Parajuli N, Seimetz M, et al. Stimulation of soluble guanylate cyclase by riociguat prevents tobacco smoke-induced pulmonary hypertension in mice [abstract]. Pneumologie. 2012;66(6):A310.
Sharkovska Y, Kalk P, Lawrenz B, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J Hypertens. 2010;28(8):1666–75.
Ott IM, Alter ML, von Websky K, et al. Effects of stimulation of soluble guanylate cyclase on diabetic nephropathy in diabetic eNOS knockout mice on top of angiotensin II receptor blockade. PLoS ONE. 2012;7(8):e42623.
Geschka S, Kretschmer A, Sharkovska Y, et al. Soluble guanylate cyclase stimulation prevents fibrotic tissue remodeling and improves survival in salt-sensitive Dahl rats. PLoS ONE. 2011;6(7):e21853.
Frey R, Muck W, Kirschbaum N, et al. Riociguat (BAY 63-2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. J Clin Pharmacol. 2011;51(7):1051–60.
Frey R, Muck W, Unger S, et al. No pharmacodynamic (PD) and pharmacokinetic (PK) interaction of riociguat (BAY 63-2521) and aspirin [abstract]. BMC Pharmacol. 2011;11(Suppl 1):P25.
Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36(4):792–9.
Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: long-term safety, tolerability and efficacy [abstract]. Am J Resp Crit Care Med. 2012;185:A2370.
Bayer HealthCare. Interim results from CHEST-2 study support benefits of Bayer’s Riociguat as demonstrated in Phase III CHEST-1 study; 2013. http://www.news.bayer.com (Accessed 27 Sep 2013).
Rubin LJ, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): a phase III long-term extension study (PATENT-2) [abstract]. Am J Resp Crit Care Med. 2013;187:A3531.
Galie N, Neuser D, Muller K, et al. A placebo-controlled, double-blind phase II interaction study to evaluate blood pressure following addition of riociguat to patients with symptomatic pulmonary arterial hypertension (PAH) receiving sildenafil (PATENT PLUS) [abstract]. Am J Resp Crit Care Med. 2013;187(Suppl):A3530.
Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41(4):853–60.
Ghio S, Bonderman D, Felix SB, et al. Left ventricular systolic dysfunction associated with pulmonary hypertension riociguat trial (LEPHT): rationale and design. Eur J Heart Fail. 2012;14(8):946–53.
Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502–11.
Bayer. Riociguat in patients with chronic thromboembolic pulmonary hypertension (CTEPH) (EAS) [ClinicalTrials.gov identifier NCT01784562]. US National Institutes of Health, ClinicalTrials.gov; 2013. http://www.clinicaltrials.gov (Accessed 8 Oct 2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Conole, D., Scott, L.J. Riociguat: First Global Approval. Drugs 73, 1967–1975 (2013). https://doi.org/10.1007/s40265-013-0149-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0149-5