Abstract
Background and Objective
The effect of renal impairment on colchicine pharmacokinetics in patients with chronic kidney disease (CKD) has not been studied previously. We evaluated the effect of renal impairment on colchicine pharmacokinetics in patients with CKD.
Methods
The pharmacokinetics and safety of a single, oral 0.6-mg dose of colchicine was evaluated in an open-label study in eight healthy subjects with normal renal function; eight subjects each with mild, moderate, or severe renal impairment; and eight subjects with end-stage renal disease (ESRD) who received a single dose prior to receiving, and again following, hemodialysis.
Results
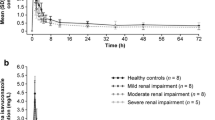
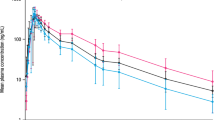
Colchicine exposure was similar for subjects with normal renal function, mild impairment, or ESRD prior to and during hemodialysis (24.7–31.7 ng·h/mL), but was up to twofold higher in subjects with moderate or severe renal impairment (48.9 and 48.0 ng·h/mL, respectively). A very small amount of the colchicine dose (mean of 5.2 %) was recovered in dialysate.
Conclusions
It appears that patients with mild or moderate renal impairment or those actively receiving hemodialysis do not show accumulation of colchicine, whereas those with severe renal impairment show a doubling of exposure. All patients with renal impairment taking colchicine should be closely monitored, especially as many patients taking colchicine often have other comorbidities and may be taking other medications.



Similar content being viewed by others
References
Kim KY, Ralph Schumacher H, Hunsche E, Wertheimer AI, Kong SX. A literature review of the epidemiology and treatment of acute gout. Clin Ther. 2003;25:1593–617.
Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep. 2002;4:252–6.
Schlesinger N. Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs. 2004;64:2399–416.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125(679–87):e671.
Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS One. 2012;7:e50046.
Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008;19:2204–10.
Food and Drug Administration. FDA approves colchicine for acute gout, mediterranean fever. Agency also provides new information to physicians regarding safe use of drug. 2009. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm174620.htm. Accessed 15 Oct 2013.
Colcrys (colchicine, USP) tablets for oral use. Full prescribing information. Deerfield, IL: Takeda Pharmaceuticals America, Inc.; 2012.
Wallace SL, Singer JZ, Duncan GJ, Wigley FM, Kuncl RW. Renal function predicts colchicine toxicity: guidelines for the prophylactic use of colchicine in gout. J Rheumatol. 1991;18:264–9.
Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349:1647–55.
El-Zawawy H, Mandell BF. Managing gout: how is it different in patients with chronic kidney disease? Cleve Clin J Med. 2010;77:919–28.
Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060–8.
Wason S, Faulkner RD, Davis MW. Are dosing adjustments required for colchicine in the elderly compared with younger patients? Adv Ther. 2012;29:551–61.
Sabouraud A, Chappey O, Dupin T, Scherrmann JM. Binding of colchicine and thiocolchicoside to human serum proteins and blood cells. Int J Clin Pharmacol Ther. 1994;32:429–32.
Tateishi T, Soucek P, Caraco Y, Guengerich FP, Wood AJ. Colchicine biotransformation by human liver microsomes. Identification of CYP3A4 as the major isoform responsible for colchicine demethylation. Biochem Pharmacol. 1997;53:111–6.
Leighton JA, Bay MK, Maldonado AL, Johnson RF, Schenker S, Speeg KV. The effect of liver dysfunction on colchicine pharmacokinetics in the rat. Hepatology. 1990;11:210–5.
Chen YJ, Huang SM, Liu CY, Yeh PH, Tsai TH. Hepatobiliary excretion and enterohepatic circulation of colchicine in rats. Int J Pharm. 2008;350:230–9.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54.
Berkun Y, Wason S, Brik R, et al. Pharmacokinetics of colchicine in pediatric and adult patients with familial Mediterranean fever. Int J Immunopathol Pharm. 2012;25:1121–30.
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER). Exposure-response relationships: study design, data analysis, and regulatory applications. 2003. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072109.pdf. Accessed 15 Oct 2014.
Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57:335–40.
Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63:2226–37.
Acknowledgments
Initial editorial assistance in the preparation of this manuscript was provided by Peter Todd, PhD, and James A. Shiffer, RPh, of Write On Time Medical Communications, LLC, who received original financial support from URL Pharma, Inc., (Philadelphia, PA, USA). Additional writing and editorial assistance was provided by Courtney Mezzacappa Zeni, PhD, and Meryl Gersh, PhD, of AlphaBioCom, LLC (King of Prussia, PA, USA) and was supported by Takeda Pharmaceuticals International, Inc., Deerfield, IL, USA.
Conflicts of interest
This study was sponsored by Mutual Pharmaceutical Company, Inc. (now part of Takeda Pharmaceuticals U.S.A., Inc., Deerfield, IL, USA). At the time of this study, Suman Wason was an employee of Mutual Pharmaceutical Company, Inc., and was part of the Takeda Pharmaceuticals U.S.A., Inc. family of companies. Robert Faulkner is a URL Pharma employee. David Mount has no financial conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Robert Faulkner and Suman Wason were working with Mutual Pharmaceutical Company, Inc., Philadelphia, PA, 19124, USA at the time of the study (now part of Takeda Pharmaceuticals U.S.A., Inc., Deerfield, IL, USA).
Rights and permissions
About this article
Cite this article
Wason, S., Mount, D. & Faulkner, R. Single-Dose, Open-Label Study of the Differences in Pharmacokinetics of Colchicine in Subjects with Renal Impairment, Including End-Stage Renal Disease. Clin Drug Investig 34, 845–855 (2014). https://doi.org/10.1007/s40261-014-0238-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0238-6