Abstract
Purpose of Review
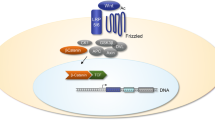
Arthritis defines a large group of diseases primarily affecting the joint. It is the leading cause of pain and disability in adults. Osteoarthritis (OA) affecting the knee or hip is the most common form among over 100 types of arthritis. Other types of arthritis include erosive hand OA, temporomandibular joint (TMJ) OA, facet joint OA, diffuse idiopathic skeletal hyperostosis (DISH), and spondyloarthritis (SpA). However, the specific molecular signals involved in the development and progression of OA and related forms of arthritis remain largely unknown. The canonical wingless/integrated (Wnt)/β-catenin signaling pathway could play a unique role in the pathogenesis of arthritis. In this review article, we will focus on the molecular mechanisms of Wnt/β-catenin signaling in the pathogenesis of OA and other types of arthritis.
Recent Findings
Emerging evidence demonstrates that Wnts and Wnt-related molecules are involved in arthritis development and progression in human genetic studies and in vitro studies. Also, mouse models have been generated to determine the role of Wnt/β-catenin signaling in the pathogenesis of arthritis.
Summary
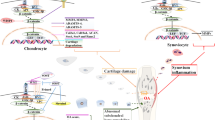
Wnt/β-catenin signaling represents a unique signaling pathway regulating arthritis development and progression, and the molecules in this particular pathway may serve as targets for the therapeutic intervention of arthritis. Mediators and downstream effectors of Wnt/β-catenin signaling are increased in OA as well other forms of arthritis, including DISH and SpA. Through extensive investigations, including pre-clinical studies in transgenic mice and translational and human studies, the Wnt/β-catenin signaling pathway has been proven to play roles in bone and joint pathology by directly affecting bone, cartilage, and synovial tissue; further, these pathologies can be reduced through targeting this pathway. Continued investigation into the distinct molecular signaling of the Wnt/β-catenin pathway will provide additional insights toward the therapeutic intervention in arthritis.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Braun J, Sieper J. Spondyloarthritiden. Z Rheumatol. 2010;69:425–34.
•• Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. The molecular mechanisms of OA initiation and progression were reviewed in this article .
Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog conductin with β-catenin, APC, and GSK3β. Science. 1998;280:596–9.
Staal FJ, Clevers H. Tcf/Lef transcription factors during T-cell development: unique and overlapping functions. Hematol J. 2000;1:3–6.
Jiang J, Struhl G. Regulation of the hedgehog and wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–6.
•• Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. This study reports, for the first time, that overexpression of β-catenin in articular cartilage of knee joint leads to OA development.
Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci U S A. 2010;107(35):15473–8.
Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, et al. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285(12):9172–9.
• Wu Q, Zhu M, Rosier RN, Zuscik MJ, O'Keefe RJ, Chen D. β-catenin, cartilage, and osteoarthritis. Ann N Y Acad Sci. 2010;1192:344–50. This review summarized the current understanding of Wnt/β-catenin signaling in cartilage during osteoarthritis development.
Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101:9757–62.
Min JL, Meulenbelt I, Riyazi N, Kloppenburg M, Houwing-Duistermaat JJ, Seymour AB, et al. Association of the frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 2005;52:1077–80.
Nakamura Y, Nawata M, Wakitani S. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am J Pathol. 2005;167(1):97–105.
Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60(2):501–12.
Honsawek S, Tanavalee A, Yuktanandana P, Ngarmukos S, Saetan N, Tantavisut S. Dickkopf-1 (Dkk-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskelet Disord. 2010;11:257.
Chan BY, Fuller ES, Russell AK, Smith SM, Smith MM, Jackson MT, et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthr Cartil. 2011;19(7):874–85.
Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis. 2014;6(2):48–57.
Bouaziz W, Funck-Brentano T, Lin H, Marty C, Ea HK, Hay E, et al. Loss of sclerostin promotes osteoarthritis in mice via beta-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24.
Roudier M, Li X, Niu QT, Pacheco E, Pretorius JK, Graham K, et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. 2013;65(3):721–31.
•• Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, Liakou C, Triantafillopoulos IK, Dontas I, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014;24(3):263–71. This review article summarized the animal models for OA research.
Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, et al. Articular cartilage and biomechanical properties of the long bones in Frzbknockout mice. Arthritis Rheum. 2007;56:4095–103.
Thysen S, Luyten FP, Lories RJ. Loss of Frzb and Sfrp1 differentially affects joint homeostasis in instability-induced osteoarthritis. Osteoarthr Cartil. 2015;23(2):275–9.
Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, et al. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251(1):142–56.
Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–87.
Chen M, Zhu M, Awad H, Li TF, Sheu TJ, Boyce BF, et al. Inhibition of β-catenin signaling causes defects in postnatal cartilage development. J Cell Sci. 2008;121(Pt 9):1455–65.
• Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, et al. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58(7):2053–64. This study reported that inhibition of canonical Wnt/β-catenin signaling could lead to defects in articular cartilage and knee joint in the Col2-ICAT transgenic mouse model.
Iwasaki LR, Crosby MJ, Marx DB, Gonzalez Y, McCall WD Jr, Ohrbach R, et al. Human temporomandibular joint eminence shape and load minimization. J Dent Res. 2010;89:722–7.
Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;35:2693–705.
• Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94(5):666–73. This review article provides current understanding of pathogenesis and treatment of TMJ OA.
•• Wang M, Li S, Xie W, Shen J, Im HJ, Holz JD, et al. Activation of β-catenin signalling leads to temporomandibular joint defects. European Cells and Materials. 2014;28:223–35. This study demonstrated that activation of β-catenin signaling in TMJ tissue could lead to OA phenotype.
Hui T, Zhao L, Zhou Y, Zhang S, Zhou Y, Liao L, Wang T, Li J, Gu J, Ye L, and Chen D (2017) Activation of β-catenin signaling in aggrecan-expressing cells in temporomandibular joint (TMJ) causes osteoarthritis-like defects. Bone Res (In press).
Yang T, Zhang J, Cao Y, Zhang M, Jing L, Jiao K, et al. Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling. J Dent Res. 2015;94(6):803–12.
Suri P, Miyakoshi A, Hunter DJ, Jarvik JG, Rainville J, Guermazi A, et al. Does lumbar spinal degeneration begin with the anterior structures? A study of the observed epidemiology in a community-based population. BMC Musculoskelet Disord. 2011;12:202.
Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000;25(23):3036–44.
•• Wang M, Tang D, Shu B, Wang B, Jin H, Hao S, et al. Conditional activation of β-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64(8):2611–23. This study demonstrates that the activation of β-catenin signaling in end-plate cartilage leads to disc degeneration.
Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology. 1976;119:559–68.
Rogers J, Waldron T. DISH and the monastic way of life. Int J Osteoarchaeol. 2001;11:357–65.
Holgate RL, Steyn M. Diffuse idiopathic skeletal hyperostosis: diagnostic, clinical, and paleopathological considerations. Clin Anat. 2016;29(7):870–7.
• Kondo N, Yuasa T, Shimono K, Tung W, Okabe T, Yasuhara R, et al. Intervertebral disc development is regulated by Wnt/β-catenin signaling. Spine (Phila Pa 1976). 2011;36(8):E513–8. This article describes the spatial-temporal expression of Wnt/β-catenin signaling during intervertebral disc development and demonstrates the role of Wnt/β-catenin signaling in disc degeneration.
Senolt L, Hulejova H, Krystufkova O, Forejtova S, Andres Cerezo L, Gatterova J, et al. Low circulating Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis. 2012;71(1):71–4.
Chou CT. How to translate basic knowledge into clinical application of biologic therapy in spondyloarthritis. Clin Dev Immunol. 2013;2013:369202.
Heiland GR, Appel H, Poddubnyy D, Zwerina J, Hueber A, Haibel H, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(4):572–4.
Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, Mueller R, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60(11):3257–62.
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–63.
Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21(6):934–45.
Baron R, Rawadi G. Minireview: targeting the Wnt/β-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–43.
•• Xie W, Zhou L, Li S, Hui T, Chen D. Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. Ann N Y Acad Sci. 2016;1364:25–31. This review article summarizes the current understanding of Wnt/β-catenin signaling in the development of spondyloarthritis.
Xing Y, Chen X, Cao Y, Huang J, Xie X, Wei Y. Expression of Wnt and Notch signaling pathways in inflammatory bowel disease treated with mesenchymal stem cell transplantation: evaluation in a rat model. Stem Cell Res Ther. 2015;22(6):101.
Serafino A, Moroni N, Zonfrillo M, Andreola F, Mercuri L, Nicotera G, et al. WNT-pathway components as predictive markers useful for diagnosis, prevention and therapy in inflammatory bowel disease and sporadic colorectal cancer. Oncotarget. 2014;5(4):978–92.
Bos CL, Diks SH, Hardwick JC, Walburg KV, Peppelenbosch MP, Richel DJ. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/β-catenin pathway activity. Carcinogenesis. 2006;27(12):2371–82.
Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, et al. Association between the abnormal expression of matrix degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–24.
Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44.
•• Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. This article demonstrates that chondrocyte-specific deletion of Mmp13 can protect DMM-induced OA development.
Sampson ER, Beck CA, Ketz J, Canary KL, Hilton MJ, Awad H, et al. Establishment of an index with increased sensitivity for assessing murine arthritis. J Orthop Res. 2011;29:1145–51.
Sampson ER, Hilton MJ, Tian Y, Chen D, Schwarz EM, Mooney RA, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93.
Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8.
Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–4.
Dao DY, Jonason JH, Zhang Y, Hsu W, Chen D, Hilton MJ, et al. Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J Bone Miner Res. 2012;27(8):1680–94.
Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, et al. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Investig. 2011;91(12):1739–52.
Wang B, Jin H, Zhu M, Li J, Zhao L, Zhang Y, et al. Chondrocyte β-catenin signaling regulates postnatal bone remodeling through modulation of osteoclast formation in a murine model. Arthritis Rheumatol. 2014;66(1):107–20.
Usui M, Xing L, Drissi H, ZusciK M, O'Keefe R, Chen D, et al. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2008;23(3):314–25.
Silvestrini G, Ballanti P, Patacchioli F, Leopizzi M, Gualtieri N, Monnazzi P, et al. Detection of osteoprotegerin (OPG) and its ligand (RANKL) mRNA and protein in femur and tibia of the rat. J Mol Hist. 2005;36:59–67.
• Wang B, Jin H, Shu B, Mira RR, Chen D. Chondrocytes-specific expression of Osteoprotegerin modulates osteoclast formation in metaphyseal bone. Sci Rep. 2015;5:13667. This article reveals that OPG expression in chondrocytes leads to mild osteopetrosis phenotype in Col2-Opg transgenic mice.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants R01 AR054465 and R01 AR070222 to DC and was also partially supported by NIH grant F31 AR070002 to JLH. This work was also partially supported by the grants from National Natural Science Foundation of China (NSFC) (grant no. 81371999) and Shenzhen Science and Technology Innovation Committee (grant no. JCYJ20160331114205502) to DC and grants from NSFC (grant no. 81301531 and 81572104) to TW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
About this article
Cite this article
Zhou, Y., Wang, T., Hamilton, J.L. et al. Wnt/β-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr Rheumatol Rep 19, 53 (2017). https://doi.org/10.1007/s11926-017-0679-z
Published:
DOI: https://doi.org/10.1007/s11926-017-0679-z