Abstract
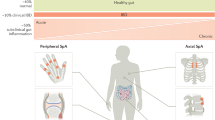
Spondylarthritides (SpA) and inflammatory bowel disease (IBD) are idiopathic, chronic inflammatory disorders. Although they are very distinct and well-defined entities, there is clinical and genetic evidence supporting some degree of overlap between the pathogenesis of the two. Subclinical gut inflammation is present in up to two thirds of all SpA patients and can evolve into IBD. This subclinical gut inflammation has been shown to be strongly associated with joint inflammation, providing a clue for a common pathophysiologic background. Despite extensive research progress in the field over the past few years, many questions remain unanswered. In this paper, we focus on the clinical, genetic, and pathophysiologic overlap of SpA and IBD. Furthermore, we discuss some of the targets that may influence therapeutic decision making.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
De Keyser F, Baeten D, Van den Bosch F, et al. Structure-modifying capacity of anti-tumour necrosis factor-alpha therapy in ankylosing spondylitis. Drugs. 2004;64(24):2793–811.
Sieper J, Rudwaleit M, Khan MA, Braun J. Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006;20(3):401–17.
Abraham C, Cho J. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–78.
Mielants H, Veys EM, Devos M, et al. The evolution of spondyloarthropathies in relation to gut histology. I. Clinical aspects. J Rheumatol. 1995;22(12):2266–72.
Mielants H, Veys EM, Cuvelier C, De Vos M, Botelberghe L. HLA-B27 related arthritis and bowel inflammation. Part 2. Ileocolonoscopy and bowel histology in patients with HLA-B27 related arthritis. J Rheumatol. 1985;12(2):294–8.
Leirisalo-Repo M, Turunen U, Stenman S, Helenius P, Seppala K. High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum. 1994;37(1):23–31.
Simenon G, Van Gossum A, Adler M, Rickaert F, Appelboom T. Macroscopic and microscopic gut lesions in seronegative spondyloarthropathies. J Rheumatol. 1990;17(11):1491–4.
Altomonte L, Zoli A, Veneziani A, et al. Clinically silent inflammatory gut lesions in undifferentiated spondyloarthropathies. Clin Rheumatol. 1994;13(4):565–70.
Mielants H, Veys E, Cuvelier C, De Vos M. Course of gut inflammation in spondylarthropathies and therapeutic consequences. Bailliere Clin Rheumatol. 1996;10(1):147–64.
Schatteman L, Mielants H, Veys EM, et al. Gut inflammation in psoriatic arthritis: a prospective ileocolonoscopic study. J Rheumatol. 1995;22(4):680–3.
Mielants H, Veys EM, Cuvelier C, et al. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy—a prospective study. J Rheumatol. 1993;20(9):1567–72.
Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23(1):29–34.
Orlando A. Gastrointestinal lesions associated with spondyloarthropathies. World J Gastroenterol. 2009;15(20):2443.
Cuvelier C, Barbatis C, Mielants H, Devos M, Roels H, Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987;28(4):394–401.
Mielants H, Veys EM, Cuvelier C, et al. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J Rheumatol. 1995;22(12):2279–84.
Felder JB, Korelitz BI, Rajapakse R, Schwarz S, Horatagis AP, Gleim G. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2000;95(8):1949–54.
Mahadevan U, Loftus Jr EV, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002;97(4):910–4.
Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(4):352–6.
Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4(2):196–202.
Reinisch W, Miehsler W, Dejaco C, et al. An open-label trial of the selective cyclo-oxygenase-2 inhibitor, rofecoxib, in inflammatory bowel disease-associated peripheral arthritis and arthralgia. Aliment Pharm Ther. 2003;17(11):1371–80.
Biancone L, Tosti C, Geremia A, et al. Rofecoxib and early relapse of inflammatory bowel disease: an open-label trial. Aliment Pharm Ther. 2004;19(7):755–64.
Sandborn WJ, Stenson WF, Brynskov J, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol. 2006;4(2):203–11.
El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective COX-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101(2):311–7.
Mielants H, Veys E. Ankylosing spondylitis and reactive arthritis: pathogenic aspects and therapeutic consequences. Ghent: Ghent University; 1988.
Dick AP, Grayson MJ, Carpenter RG, Petrie A. Controlled trial of sulphasalazine in the treatment of ulcerative colitis. Gut. 1964;5:437–42.
Mielants H, Schatteman G, Gyselbrecht L, Elewaut D. Influence of sulphasalazine on the evolution of gut inflammation and locomotor manifestations in the spondylarthropathies. Br J Rheumatol. 1995;34(s1):115 (abstract n220).
Chen J, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2005(2):CD004800. doi:10.1002/14651858.CD004800.pub2
Dougados M, vam der Linden S, Leirisalo-Repo M, et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38(5):618–27.
Benitez-Del-Castillo JM, Garcia-Sanchez J, Iradier T, Banares A. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye (Lond). 2000;14(Pt 3A):340–3.
Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119(6):1461–72.
Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119(6):1473–82.
Faustini F, Zoli A, Ferraccioli GF. Immunologic and genetic links between spondylarthropathies and inflammatory bowel diseases. Eur Rev Med Pharmacol Sci. 2009;13:1–9.
Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15(46):5784–8.
Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58(8):2307–17.
Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–3.
Rahman P, Inman RD, Gladman DD, Reeve JP, Peddle L, Maksymowych WP. Association of interleukin-23 receptor variants with ankylosing spondylitis. Arthritis Rheum. 2008;58(4):1020–5.
Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351(20):2069–79.
Shale M, Ghosh S. Beyond TNF, Th1 and Th2 in inflammatory bowel disease. Gut. 2008;57(10):1349–51.
Leso V, Leggio L, Armuzzi A, Gasbarrini G, Gasbarrini A, Addolorato G. Role of the tumor necrosis factor antagonists in the treatment of inflammatory bowel disease: an update. Eur J Gastroen Hepat. 2010;22(7):779–86.
Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–98.
Inman RD, Davis Jr JC, van der Heijde D, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58(11):3402–12.
Davis Jr JC, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48(11):3230–6.
van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52(2):582–91.
van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54(7):2136–46.
van der Horst-Bruinsma IE, van Denderen JC, Visman I, Suttorp-Schulten M, Dijkmans B, Nurmohamed MT. Decreased recurrence rate of anterior uveitis in ankylosing spondylitis treated with adalimumab—an interim analysis [abstract 1933]. Presented at the ACR/ARHP Scientific Meeting 2010. Atlanta, GA; November 6–11, 2010.
Heiligenhaus A, Thurau S, Hennig M, Grajewski RS, Wildner G. Anti-inflammatory treatment of uveitis with biologicals: new treatment options that reflect pathogenetic knowledge of the disease. Graefes Arch Clin Exp Ophthalmol. 2010;248(11):1531–51.
Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60(4):976–86.
Ortonne JP, Tassel C, Reich K. Efficacy of certolizumab pegol, a PEGylated Fab’ fragment of an anti-TNF-a monoclonal antibody, in patients previously exposed to biologicals. Preliminary results of a randomised, placebo-controlled phase II clinical trial in psoriasis. Presented at the 16th Congress of the European Academy of Dermatology and Venerology, Vienna, Austria 16–20 May 2007. 2007.
Marzo-Ortega H, McGonagle D, O’Connor P, Emery P. Efficacy of etanercept for treatment of Crohn’s related spondyloarthritis but not colitis. Ann Rheum Dis. 2003;62(1):74–6.
Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121(5):1088–94.
Breban M. Genetics of spondyloarthritis. Best Pract Res Clin Rheumatol. 2006;20(3):593–9.
Brown MA. Genetics of ankylosing spondylitis. Curr Opin Rheumatol. 2010;22(2):126–32.
Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–3.
Jacques P, Elewaut D. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. 2008;1(5):364–71.
•• Danoy P, Pryce K, Hadler J, et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genetics. 2010;6(12):e1001195. This paper provides evidence for additional shared genetic associations between AS and IBD and confirms the importance of the Th17 lymphocyte subset in the pathogenesis of AS.
Francois RJ, Neure L, Sieper J, Braun J. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis. 2006;65(6):713–20.
Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, Yorgancioglu R. Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol. 2007;26(2):211–5.
Visvanathan S, Wagner C, Marini JC, et al. Inflammatory biomarkers, disease activity and spinal disease measures in patients with ankylosing spondylitis after treatment with infliximab. Ann Rheum Dis. 2008;67(4):511–7.
Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54(9):2817–29.
Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126(4):989–96. discussion 47.
Tanaka T, Kuwahara Y, Shima Y, et al. Successful treatment of reactive arthritis with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Arthritis Rheum. 2009;61(12):1762–4.
Brulhart L, Nissen MJ, Chevallier P, Gabay C. Tocilizumab in a patient with ankylosing spondylitis and Crohn’s disease refractory to TNF antagonists. Joint, bone, spine: revue du rhumatisme 2010.
• Baeten D, Sieper J, Emery P, et al. The anti-IL17A monoclonal antibody secukinumab (AIN457) showed good safety and efficacy in the treatment of active ankylosing spondylitis [abstract L7]. Presented at the ACR/ARHP Scientific Meeting 2010. Atlanta, GA; November 6–11, 2010. These results, although they are interim results, suggest a major role for the Th17 pathway as a new therapeutic target for AS in the very near future.
MacDonald JK, McDonald JW. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database of Systematic Reviews. 2007(1):CD006097. doi:10.1002/14651858.CD006097.pub2
Elewaut D, De Keyser F, Van Den Bosch F, et al. Enrichment of T cells carrying beta7 integrins in inflamed synovial tissue from patients with early spondyloarthropathy, compared to rheumatoid arthritis. J Rheumatol. 1998;25(10):1932–7.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Praet, L., Van den Bosch, F., Mielants, H. et al. Mucosal Inflammation in Spondylarthritides: Past, Present, and Future. Curr Rheumatol Rep 13, 409–415 (2011). https://doi.org/10.1007/s11926-011-0198-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-011-0198-2