Abstract
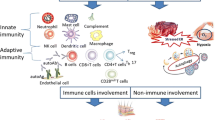
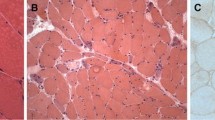
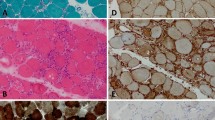
The idiopathic inflammatory myopathies (IIM) are systemic autoimmune diseases that have predominant mononuclear inflammatory cell infiltrates in the skeletal muscle. The cells that are typically involved in the pathogenesis of disease are B-lymphocytes, T-lymphocytes, macrophages, dendritic cells, and natural killer cells. However, in addition to these immune cells, cells of nonimmunologic origin, such as myocytes, may be directly involved in the immune response. The local milieu also consists of distinct cytokine and chemokine profiles considered related to type 1 interferon stimulation. Tumor necrosis factor and interleukin 1 are also prominent, proinflammatory cytokines involved in the evolution of IIM. Although the pathologic processes involved in IIM have yet to be fully elucidated, we understand the inflammatory milieu is a model of dynamic flux made of diverse cytokine and chemokine expressions leading to alterations in muscle fiber structure and function.
Similar content being viewed by others
References and Recommended Reading
Feldman BM, Rider LG, Reed AM, Pachman LM: Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet 2008, 371:2201–2212.
Dalakas MC, Hohlfeld R: Polymyositis and dermatomyositis. Lancet 2003, 362:971–982.
Dalakas MC, Sivakumar K: The immunopathologic and inflammatory differences between dermatomyositis, polymyositis and sporadic inclusion body myositis. Curr Opin Neurol 1996, 9:235–239.
Page G, Chevrel G, Miossec P: Anatomic localization of immature and mature dendritic cell subsets in dermatomyositis and polymyositis: interaction with chemokines and Th1 cytokine-producing cells. Arthritis Rheum 2004, 50:199–208.
Hohlfeld R, Goebels N, Engel AG: Cellular mechanisms in inflammatory myopathies. Baillieres Clin Neurol 1993, 2:617–635.
Whitaker JN, Engel WK: Vascular deposits of immunoglobulin and complement in idiopathic inflammatory myopathy. N Engl J Med 1972, 286:333–338.
López de Padilla CM, Vallejo AN, Lacomis D, et al.: Extra-nodal lymphoid microstructures in inflamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum 2009, 60:1160–1172.
Engel AG, Arahata K: Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol 1986, 17:704–721.
De Bleecker JL, Engel AG, Butcher EC: Peripheral lymphoid tissue-like adhesion molecule expression in nodular infiltrates in inflammatory myopathies. Neuromuscul Disord 1996, 6:255–260.
Lopez de Padilla CM, Vallejo AN, McNallan KT, et al.: Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum 2007, 56:1658–1668.
Greenberg SA, Pinkus JL, Pinkus GS, et al.: Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol 2005, 57:664–678.
Greenberg SA, Pinkus GS, Amato AA, Pinkus JL: Myeloid dendritic cells in inclusion-body myositis and polymyositis. Muscle Nerve 2007, 35:17–23.
Tesmer LA, Lundy SK, Sarkar S, Fox DA: Th17 cells in human disease. Immunol Rev 2008, 223:87–113.
Bettelli E, Korn T, Oukka M, Kuchroo VK: Induction and effector functions of T(H)17 cells. Nature 2008, 453:1051–1057.
Dong C: TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 2008, 8:337–348.
Ikezoe K, Ohshima S, Osoegawa M, et al.: Expression of granulysin in polymyositis and inclusion-body myositis. J Neurol Neurosurg Psychiatry 2006, 77:1187–1190.
Page G, Sattler A, Kersten S, et al.: Plasma cell-like morphology of Th1-cytokine-producing cells associated with the loss of CD3 expression. Am J Pathol 2004, 164:409–417.
Chevrel G, Page G, Granet C, et al.: Interleukin-17 increases the effects of IL-1 beta on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J Neuroimmunol 2003, 137:125–133.
Chevrel G, Granet C, Miossec P: Contribution of tumour necrosis factor alpha and interleukin (IL) 1beta to IL6 production, NF-kappaB nuclear translocation, and class I MHC expression in muscle cells: in vitro regulation with specific cytokine inhibitors. Ann Rheum Dis 2005, 64:1257–1262.
De Bleecker JL, Engel AG: Immunocytochemical study of CD45 T cell isoforms in inflammatory myopathies. Am J Pathol 1995, 146:1178–1187.
Colonna M, Trinchieri G, Liu YJ: Plasmacytoid dendritic cells in immunity. Nat Immunol 2004, 5:1219–1226.
Griffin TA, Reed AM: Pathogenesis of myositis in children. Curr Opin Rheumatol 2007, 19:487–491.
Baechler EC, Bauer JW, Slattery CA, et al.: An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol Med 2007, 13:59–68.
Greenberg SA, Bradshaw EM, Pinkus JL, et al.: Plasma cells in muscle in inclusion body myositis and polymyositis. Neurology 2005, 65:1782–1787.
Arahata K, Engel AG: Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol 1984, 16:193–208.
Bradshaw EM, Orihuela A, McArdel SL, et al.: A local antigen-driven humoral response is present in the inflammatory myopathies. J Immunol 2007, 178:547–556.
Rostasy KM, Piepkorn M, Goebel HH, et al.: Monocyte/macrophage differentiation in dermatomyositis and polymyositis. Muscle Nerve 2004, 30:225–230.
Confalonieri P, Bernasconi P, Megna P, et al.: Increased expression of beta-chemokines in muscle of patients with inflammatory myopathies. J Neuropathol Exp Neurol 2000, 59:164–169.
De Paepe B, De Bleecker JL: Beta-chemokine receptor expression in idiopathic inflammatory myopathies. Muscle Nerve 2005, 31:621–627.
Civatte M, Bartoli C, Schleinitz N, et al.: Expression of the beta chemokines CCL3, CCL4, CCL5 and their receptors in idiopathic inflammatory myopathies. Neuropathol Appl Neurobiol 2005, 31:70–79.
Rostasy KM, Schmidt J, Bahn E, et al.: Distinct inflammatory properties of late-activated macrophages in inflammatory myopathies. Acta Myol 2008, 27:49–53.
Arahata K, Engel AG: Monoclonal antibody analysis of mononuclear cells in myopathies. IV: cell-mediated cytotoxicity and muscle fiber necrosis. Ann Neurol 1988, 23:168–173.
Mitsuo A, Morimoto S, Nakiri Y, et al.: Decreased CD161+CD8+ T cells in the peripheral blood of patients suffering from rheumatic diseases. Rheumatology (Oxford) 2006, 45:1477–1484.
O’Gorman MR, Bianchi L, Zaas D, et al.: Decreased levels of CD54 (ICAM-1)-positive lymphocytes in the peripheral blood in untreated patients with active juvenile dermatomyositis. Clin Diagn Lab Immunol 2000, 7:693–697.
McNallan K WM, Crowson C, Reed AM: Absence of killer Ig-like inhibitory receptor with the associated HLA ligand in juvenile dermatomyositis. Arthritis Rheum 2007, 56:9S.
Li CK, Varsani H, Holton JL, et al.: MHC class I overex-pression on muscles in early juvenile dermatomyositis. J Rheumatol 2004, 31:605–609.
Nagaraju K: Role of major histocompatibility complex class I molecules in autoimmune myositis. Curr Opin Rheumatol 2005, 17:725–730.
van der Pas J, Hengstman GJ, ter Laak HJ, et al.: Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry 2004, 75:136–139.
Wiendl H, Mitsdoerffer M, Hofmeister V, et al.: The non-classical MHC molecule HLA-G protects human muscle cells from immune-mediated lysis: implications for myoblast transplantation and gene therapy. Brain 2003, 126(Pt 1):176–185.
Wiendl H, Mitsdoerffer M, Schneider D, et al.: Human muscle cells express a B7-related molecule, B7-H1, with strong negative immune regulatory potential: a novel mechanism of counterbalancing the immune attack in idiopathic inflammatory myopathies. Faseb J 2003, 17:1892–1894.
Nagaraju K, Casciola-Rosen L, Lundberg I, et al.: Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum 2005, 52:1824–1835.
Behrens L, Bender A, Johnson MA, Hohlfeld R: Cytotoxic mechanisms in inflammatory myopathies. Co-expression of Fas and protective Bcl-2 in muscle fibres and inflammatory cells. Brain 1997, 120(Pt 6):929–938.
Tews DS, Goebel HH: Cell death and oxidative damage in inflammatory myopathies. Clin Immunol Immunopathol 1998, 87:240–247.
Ulfgren AK, Grundtman C, Borg K, et al.: Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum 2004, 50:1586–1594.
Lundberg I, Kratz AK, Alexanderson H, Patarroyo M: Decreased expression of interleukin-1alpha, interleukin-1beta, and cell adhesion molecules in muscle tissue following corticosteroid treatment in patients with polymyositis and dermatomyositis. Arthritis Rheum 2000, 43:336–348.
Casciola-Rosen L, Nagaraju K, Plotz P, et al.: Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med 2005, 201:591–601.
Theofilopoulos AN, Baccala R, Beutler B, Kono DH: Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 2005, 23:307–336.
Eloranta ML, Barbasso Helmers S, Ulfgren AK, et al.: A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum 2007, 56:3112–3124.
Tezak Z, Hoffman EP, Lutz JL, et al.: Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol 2002, 168:4154–4163.
Raju R, Dalakas MC: Gene expression profile in the muscles of patients with inflammatory myopathies: effect of therapy with IVIg and biological validation of clinically relevant genes. Brain 2005, 128(Pt 8):1887–1896.
Wenzel J, Schmidt R, Proelss J, et al.: Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol 2006, 31:576–582.
O’Connor KA, Abbott KA, Sabin B, et al.: MxA gene expression in juvenile dermatomyositis peripheral blood mononuclear cells: association with muscle involvement. Clin Immunol 2006, 120:319–325.
Baechler EC, Batliwalla FM, Reed AM, et al.: Gene expression profiling in human autoimmunity. Immunol Rev 2006, 210:120–137.
Walsh RJ, Kong SW, Yao Y, et al.: Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum 2007, 56:3784–3792.
Bilgic H YS, Ytterberg S, McNallan KT, et al.: IL-6 and IFN-regulated genes and chemokines as biomarkers of disease activity in dermatomyositis. Arthritis Rheum 2009, in press.
Sugiura T, Harigai M, Kawaguchi Y, et al.: Increased IL-15 production of muscle cells in polymyositis and dermatomyositis. Int Immunol 2002, 14:917–924.
De Bleecker JL, De Paepe B, Vanwalleghem IE, Schroder JM: Differential expression of chemokines in inflammatory myopathies. Neurology 2002, 58:1779–1785.
Fall N, Bove KE, Stringer K, et al.: Association between lack of angiogenic response in muscle tissue and high expression of angiostatic ELR-negative CXC chemokines in patients with juvenile dermatomyositis: possible link to vasculopathy. Arthritis Rheum. 2005, 52:3175–3180.
Michaelis D, Goebels N, Hohlfeld R: Constitutive and cytokine-induced expression of human leukocyte antigens and cell adhesion molecules by human myotubes. Am J Pathol 1993, 143:1142–1149.
Tews DS, Goebel HH: Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol 1996, 55:342–347.
Tateyama M, Nagano I, Yoshioka M, et al.: Expression of tumor necrosis factor-alpha in muscles of polymyositis. J Neurol Sci 1997, 146:45–51.
Fedczyna TO, Lutz J, Pachman LM: Expression of TNFalpha by muscle fibers in biopsies from children with untreated juvenile dermatomyositis: association with the TNFalpha-308A allele. Clin Immunol 2001, 100:236–239.
Pachman LM, Liotta-Davis MR, Hong DK, et al.: TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum 2000, 43:2368–2377.
De Bleecker JL, Meire VI, Declercq W, Van Aken EH: Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromuscul Disord 1999, 9:239–246.
Dorph C, Englund P, Nennesmo I, Lundberg IE: Signs of inflammation in both symptomatic and asymptomatic muscles from patients with polymyositis and dermatomyositis. Ann Rheum Dis 2006, 65:1565–1571.
Lepidi H, Frances V, Figarella-Branger D, et al.: Local expression of cytokines in idiopathic inflammatory myopathies. Neuropathol Appl Neurobiol 1998, 24:73–79.
Grundtman C, Salomonsson S, Dorph C, et al.: Immunolocalization of interleukin-1 receptors in the sarcolemma and nuclei of skeletal muscle in patients with idiopathic inflammatory myopathies. Arthritis Rheum 2007, 56:674–687.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reed, A.M., Ernste, F. The inflammatory milieu in idiopathic inflammatory myositis. Curr Rheumatol Rep 11, 295–301 (2009). https://doi.org/10.1007/s11926-009-0041-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-009-0041-1