Abstract
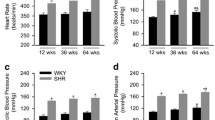
Angiotensin type 1a receptor (AT1aR) is thought to play an important role in the development of hypertension. However, it is unknown how the AT1aR expression in vascular tissue is changed during the development of hypertension or if the degree of methylation in the AT1aR promoter correlates with the expression of AT1aR. To address these questions, we measured AT1aR mRNA, protein expression, and methylation status of the AT1aR promoter in the aorta and mesenteric artery of male spontaneously hypertensive rats (SHRs) and age-matched Wistar-Kyoto (WKY) rats acting as controls at pre-hypertensive (4 weeks), evolving (10 weeks), and established (20 weeks) stages of hypertension. The expression of the AT1aR mRNA and protein was not different between the SHRs and WKY rats at 4 weeks. However, they were significantly greater in SHRs than in WKY rats at 20 weeks. Bisulfite sequencing revealed that the AT1aR promoter from the aorta and mesenteric artery of the SHRs was progressively hypo-methylated with age as compared with their WKY rat counterparts. These results suggest that the heightened AT1aR expression in SHRs is related to the AT1aR promoter hypo-methylation, which might be a consequence of the increased blood pressure and may be important in the maintenance of high blood pressure.


Similar content being viewed by others
References
Unger T, Paulis L, Sica DA (2011) Therapeutic perspectives in hypertension: novel means for renin-angiotensin-aldosterone system modulation and emerging device-based approaches. Eur Heart J 32(22):2739–2747
Bader M (2010) Tissue renin-angiotensin-aldosterone systems: targets for pharmacological therapy. Annu Rev Pharmacol Toxicol 50:439–465
Savoia C, Burger D, Nishigaki N et al (2011) Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med 13:e11
Navar LG, Prieto MC, Satou R et al (2011) Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11(2):180–186
Dasgupta C, Zhang L (2011) Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov Today 16(1–2):22–34
Aplin M, Bonde MM, Hansen JL (2009) Molecular determinants of angiotensin II type 1 receptor functional selectivity. J Mol Cell Cardiol 46(1):15–24
Higuchi S, Ohtsu H, Suzuki H et al (2007) Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 112(8):417–428
Nguyen Dinh Cat A, Touyz RM (2011) Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep 13(2):122–128
Kitami Y, Okura T, Marumoto K et al (1992) Differential gene expression and regulation of type-1 angiotensin II receptor subtypes in the rat. Biochem Biophys Res Commun 188(1):446–452
Llorens-Cortes C, Greenberg B, Huang H et al (1994) Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension 24(5):538–548
Gasc JM, Shanmugam S, Sibony M et al (1994) Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension 24(5):531–537
Du Y, Guo DF, Inagami T et al (1996) Regulation of ANG II-receptor subtype and its gene expression in adrenal gland. Am J Physiol 271(2 Pt 2):H440–H446
Nguyen Dinh Cat A, Montezano AC, Burger D et al (2013) Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19(10):1110–1120
Imaizumi S, Miura S, Yahiro E et al (2013) Class- and molecule-specific differential effects of angiotensin II type 1 receptor blockers. Curr Pharm Des 19(17):3002–3008
Frey FJ (2005) Methylation of CpG islands: potential relevance for hypertension and kidney diseases. Nephrol Dial Transplant 20(5):868–869
Bogdarina I, Welham S, King PJ et al (2007) Epigenetic modification of the renin- angiotensin system in the fetal programming of hypertension. Circ Res 100(4):520–526
Friso S, Pizzolo F, Choi SW et al (2008) Epigenetic control of 11 beta- hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis 199(2):323–327
Smolarek I, Wyszko E, Barciszewska AM et al (2010) Global DNA methylation changes in blood of patients with essential hypertension. Med Sci Monit 16(3):CR149–CR155
Cho HM, Lee HA, Kim HY et al (2011) Expression of Na+–K+–2Cl– cotransporter 1 is epigenetically regulated during postnatal development of hypertension. Am J Hypertens 24(12):1286–1293
Bellavia A, Urch B, Speck M et al (2013) DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J Am Heart Assoc 2(3):e000212
Wang X, Falkner B, Zhu H et al (2013) A genome-wide methylation study on essential hypertension in young African American males. PLoS One 8(1):e53938
Wang F, Demura M, Cheng Y et al (2014) Dynamic CCAAT/enhancer binding protein-associated changes of DNA methylation in the angiotensinogen gene. Hypertension 63(2):281–288
Meissner A, Mikkelsen TS, Gu H et al (2008) Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454(7205):766–770
Jones PA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13(7):484–492
Deaton AM, Bird A (2011) CpG islands and the regulation of transcription. Genes Dev 25(10):1010–1022
Maunakea AK, Nagarajan RP, Bilenky M et al (2010) Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 466(7303):253–257
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Viswanathan M, Tsutsumi K, Correa FM et al (1991) Changes in expression of angiotensin receptor subtypes in the rat aorta during development. Biochem Biophys Res Commun 179(3):1361–1367
Millis RM (2011) Epigenetics and hypertension. Curr Hypertens Rep 13(1):21–28
Pojoga LH, Williams JS, Yao TM et al (2011) Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol 301(5):H1862–H1871
Chu CH, Lo JF, Hu WS et al (2012) Histone acetylation is essential for ANG-II-induced IGF-IIR gene expression in H9c2 cardiomyoblast cells and pathologically hypertensive rat heart. J Cell Physiol 227(1):259–268
Lee HA, Lee DY, Lee HJ et al (2012) Enrichment of (pro)renin receptor promoter with activating histone codes in the kidneys of spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst 13(1):11–18
Lee HA, Cho HM, Lee DY et al (2012) Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension 59(3):621–626
Batkai S, Thum T (2012) MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep 14(1):79–87
Bird A (2007) Perceptions of epigenetics. Nature 447(7143):396–398
Marx V (2012) Epigenetics: reading the second genomic code. Nature 491(7422):143–147
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81200193 and No. 81273507).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fang Pei and Xinquan Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pei, F., Wang, X., Yue, R. et al. Differential expression and DNA methylation of angiotensin type 1A receptors in vascular tissues during genetic hypertension development. Mol Cell Biochem 402, 1–8 (2015). https://doi.org/10.1007/s11010-014-2295-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2295-9