Abstract
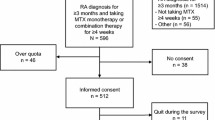
Methotrexate (MTX) has become the first-line treatment for rheumatoid (RA) and psoriatic arthritis (PsA); however, few studies have focused on its tolerability. The objective of our analyses was to study RA and PsA patients in whom MTX was discontinued, the reasons for this and the duration of MTX treatment prior to withdrawal. A retrospective electronic database review was undertaken to identify all patients who had received MTX for RA or PsA. Patients who had discontinued MTX were then identified, and the reasons for this were categorised. The duration of MTX treatment was assessed in those who had stopped treatment due to intolerability. A total of 1,257 patients who had received MTX were identified [762 (61 %) RA and 193 (15 %) PsA]. MTX had been stopped in 260 (34 %) patients with RA and 71 (36 %) patients with PsA most commonly due to gastrointestinal intolerability. The median duration of MTX treatment was 10 months in both groups, mean duration 21 and 18.6 months in RA and PsA groups, respectively. Overall, one third of patients with RA and PsA stop MTX most commonly due to poor tolerability. In the context of chronic disease, the median duration of treatment is short (10 months). Our analysis did not include patients who suffer from side effects but continue therapy; thus, the magnitude of the problem may be substantially greater therefore as poor tolerability impacts treatment adherence.


Similar content being viewed by others
References
Bathon J, Robles M, Ximenes AC, Nayiager S et al (2011) Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann Rheum Dis 70:1949–1956
Schiff M, Keiserman M, Codding C et al (2008) Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 67:1096–1103
Lu LJ, Bao CD, Dai M et al (2009) Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum 61:979–987
Smolen J, Landewe RB, Mease P et al (2009) Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 68:797–804
van de Heijde D, Klareskog L, Rodriguez-Valverde V et al (2006) Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 54:1063–1074
Salliot C, van der Heijde D (2009) Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 68:1100–1104
Katchamart W, Trudeau J, Phumethum V et al (2010) Methotrexate monotherapy versus methotrexate combination therapy with non-biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Cochrane Database Syst Rev 14(4):CD008495
Bologna C, Jorgensen C, Sany J (1997) Methotrexate as the initial second-line disease modifying agent in the treatment of rheumatoid arthritis patients. Cl Exp Rheum 15:597–601
Keysser M, Keysser G, Keysser C (1999) Long-term application of disease modifying antirheumatic drugs (DMARD). A single-center, observational study of 1681 patients with rheumatoid arthritis (RA). Z Rheumatol 58:267–276
Emery P, Breedveld FC, Lemmel EM et al (2000) A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology 39:655–665
van Ede AE, Laan RF, Rood MJ et al (2001) Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 44:1515–1524
Smolen JS, Aletaha D, Bijlsma JWJ et al (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69:631–637
Gäwert L, Hierse F, Zink A et al (2011) How well do patient reports reflect adverse drug reactions reported by rheumatologists? Agreement of physician- and patient-reported adverse events in patients with rheumatoid arthritis observed in the German biologics register. Rheumatology 50:152–160
Shiroky JB, Neville C, Esdaile JM et al (1993) Low-dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis. Results of a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum 36:795–803
Caro JJ, Salas M, Speckman JL et al (1999) Persistence with treatment for hypertension in actual practice. CMAJ 160:31–37
Mariette X, Cazals-Hatem D, Warszawki J et al (2002) Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood 99:3909–3915
Owen SA, Lunt M, Bowes J, et al (2011) MTHFR gene polymorphisms and outcome of methotrexate treatment in patients with rheumatoid arthritis: analysis of key polymorphisms and meta-analysis of C677T and A1298C polymorphisms. Pharmacogenomics J 13:137–147
Lee YH, Song GG (2010) Associations between the C677T and A1298C polymorphisms of MTHFR and the efficacy and toxicity of methotrexate in rheumatoid arthritis: a meta-analysis. Clin Drug Investig 30:101–108
Fisher MC, Cronstein BN (2011) Metaanalysis of methylenetetrahydrofolate reductase (MTHFR) polymorphisms affecting methotrexate toxicity. J Rheumatol 36:539–545
Urano W, Taniguchi A, Yamanaka H et al (2002) Polymorphisms in the methylenetetrahydrofolate reductase gene were associated with both the efficacy and the toxicity of methotrexate used for the treatment of rheumatoid arthritis, as evidenced by single locus and haplotype analyses. Pharmacogenetics 12:183–190
Cáliz R, del Amo J, Balsa A et al (2012) The C677T polymorphism in the MTHFR gene is associated with the toxicity of methotrexate in a Spanish rheumatoid arthritis population. Scand J Rheumatol 41:10–14
Weisman MH, Furst DE, Park GS et al (2006) Risk genotypes in folate-dependent enzymes and their association with methotrexate-related side effects in rheumatoid arthritis. Arthritis Rheum 54:607–612
Berkun Y, Levartovsky D, Rubinow A et al (2004) Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Ann Rheum Dis 63:1227–1231
Spyridopoulou KP, Dimou NL, Hamodrakas SJ et al (2012) Methylene tetrahydrofolate reductase gene polymorphisms and their association with methotrexate toxicity: a meta-analysis. Pharmacogenet Genomics 22:117–133
Wluka A, Buchbinder R, Mylvaganam A et al (2000) Longterm methotrexate use in rheumatoid arthritis: 12 year follow up of 460 patients treated in community practice. J Rheumatol 27:1864–1871
Funding
The study was supported by the charity CARE (Cambridge Arthritis Research Endeavour).
Disclosures
Elena Nikiphorou, Andra Negoescu, John D Fitzpatrick, Calum Goudie, Andrew Badcock, Andrew JK Östör and Anshuman P Malaviya have no disclosures to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Significance and innovation
• Over one third of patients with RA and PsA stop methotrexate in daily practice.
• The most common reason for stopping methotrexate is intolerability.
• The median duration of MTX treatment is 10 months.
• Methotrexate side effects should be actively sort to optimise management.
Rights and permissions
About this article
Cite this article
Nikiphorou, E., Negoescu, A., Fitzpatrick, J.D. et al. Indispensable or intolerable? Methotrexate in patients with rheumatoid and psoriatic arthritis: a retrospective review of discontinuation rates from a large UK cohort. Clin Rheumatol 33, 609–614 (2014). https://doi.org/10.1007/s10067-014-2546-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2546-x