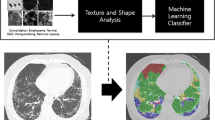
Abstract
Objectives
The Scleroderma Lung Study showed the efficacy of cyclophosphamide in modestly improving the forced vital capacity (FVC) compared with placebo over 1 year. Using changes in texture-based scores that quantify lung fibrosis as the percentage involvement of reticulation patterns, the effectiveness of cyclophosphamide was re-assessed by examining its impact on quantitative lung fibrosis (QLF).
Methods
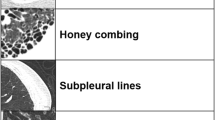
Axial HRCT images were acquired (1-mm slice thickness, 10-mm increments) in the prone position at inspiration. A validated model for quantifying interstitial disease patterns was applied to images from 83 subjects at baseline and 12 months. Scores were calculated for six zones (upper, mid, lower of the right/left lung) and the whole lung. Average changes were compared. Correlations were performed between QLF and physiological and clinical scores.
Results
From the most severe zones identified at baseline, QLF scores decreased by 2.6% in the cyclophosphamide group, whereas they increased by 9.1% in the placebo group, leading to ~12% difference (p = 0.0027). Between-treatment difference in whole lung QLF was ~5% (p = 0.0190). Significant associations were observed between changes in QLF and FVC (r = −0.33), dyspnea score (r = −0.29), and consensus visual score (p = 0.0001).
Conclusions
QLF scores provide an objective quantitative tool for assessing treatment efficacy in scleroderma-related interstitial lung disease.





Similar content being viewed by others
References
Steen VD, Conte C, Owens GR, Medsger TA Jr (1994) Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 37:1283–1289
Steen VD, Medsger TA Jr (2000) Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 43:2437–2444
Medsger TA Jr (2004) Classification, prognosis. In: Clements PJ, Furst DE (eds) Systemic Sclerosis. Lippincott, Williams and Wilkins, Philadelphia, pp 17–28
Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, Haslam PL, Vassilakis DA, Black CM, du Bois RM (2002) Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 165:1581–1586
Kim DS, Yoo B, Lee JS, Kim EK, Lim CM, Lee SD, Koh Y, Kim WS, Kim WD, Colby TV, Kitiaichi M (2002) The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis 19:121–127
Minai OA, Dweik RA, Arroliga AC (1998) Manifestations of scleroderma pulmonary disease. Clin Chest Med 19:713–731
Fischer A, Swigris JJ, Groshong SD, Cool CD, Sahin H, Lynch DA, Curran-Everett D, Gillis JZ, Meehan RT, Brown KK (2008) Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest 134:601–605
Kim TS, Lee KS, Chung MP, Han J, Park JS, Hwang JH, Kwon OJ, Rhee CH (1998) Nonspecific interstitial pneumonia with fibrosis: high-resolution CT and pathologic findings. AJR Am J Roentgenol 171:1645–1650
Johkoh T, Muller NL, Colby TV, Ichikado K, Taniguchi H, Kondoh Y, Fujimoto K, Kinoshita M, Arakawa H, Yamada H, Suga M, Ando M, Koyama M, Nakamura H (2002) Nonspecific interstitial pneumonia: correlation between thin-section CT findings and pathologic subgroups in 55 patients. Radiology 225:199–204
Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, Roberts C, Desai S, Herrick AL, McHugh NJ, Foley NM, Pearson SB, Emery P, Veale DJ, Denton CP, Wells AU, Black CM, du Bois RM (2006) A multicenter, prospective, randomized, double-blind, placebo-controlled trial of treatment with corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 54:3962–3970
Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M, Scleroderma Lung Study Research Group (2006) Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354:2655–2666
Goldin J, Elashoff R, Kim HJ, Yan X, Lynch D, Strollo D, Roth MD, Clements P, Furst DE, Khanna D, Vasunilashorn S, Li G, Tashkin DP (2009) Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest 136:1333–1340
Martinez FJ, McCune WJ (2006) Cyclophosphamide for scleroderma lung disease. N Engl J Med 354:2707–2709
Morgenthau AS, Padilla ML (2009) Spectrum of fibrosing diffuse parenchymal lung disease. Mt Sinai J Med 76:2–23
Kim HJ, Li G, Gjertson D, Elashoff R, Shah SK, Ochs R, Vasunilashorn F, Abtin F, Brown MS, Goldin JG (2008) Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol 15:1004–1016
Kim HJ, Tashkin DP, Clements PJ, Li G, Brown MS, Elashoff R, Gjertson DW, Abtin F, Lynch DA, Strollo DC, Goldin JG (2010) A computer-aided diagnosis system for quantitative scoring of extent of lung fibrosis in scleroderma patients. Clin Exp Rheumatol 5:S26–S35
Collins CD, Wells AU, Hansell DM, Morgan RA, MacSweeney JE, du Bois RM, Rubens MB (1994) Observer variation in pattern type and extent of disease in fibrosing alveolitis on thin section computed tomography and chest radiography. Clin Radiol 49:236–240
Camiciottoli G, Orlandi I, Bartolucci M, Meoni E, Nacci F, Diciotti S, Barcaroli C, Conforti ML, Pistolesi M, Matucci-Cerinic M, Mascalchi M (2007) Lung CT densitometry in systemic sclerosis: correlation with lung function, exercise testing, and quality of life. Chest 131:672–681
Lynch DA (2007) Quantitative CT of fibrotic interstitial lung disease. Chest 131:643–644
Sumikawa H, Johkoh T, Yamamoto S, Yanagawa M, Inoue A, Honda O, Yoshida S, Tomiyama N, Nakamura H (2009) Computed tomography values calculation and volume histogram analysis for various computed tomographic patterns of diffuse lung diseases. J Comput Assist Tomogr 33:731–738
Uppaluri R, Hoffman EA, Sonka M, Hartley PG, Hunninghake GW, McLennan G (1999) Computer recognition of regional lung disease patterns. Am J Respir Crit Care Med 160:648–654
Chabat F, Yang GZ, Hansell DM (2003) Obstructive lung diseases: texture classification for differentiation at CT. Radiology 228:871–877
Boehm HF, Fink C, Attenberger U, Becker C, Behr J, Reiser M (2008) Automated classification of normal and pathologic pulmonary tissue by topological texture features extracted from multi-detector CT in 3D. Eur Radiol 18:2745–2755
Depeursinge A, Iavindrasana J, Hidki A, Cohen G, Geissbuhler A, Platon A, Poletti PA, Muller H (2010) Comparative performance analysis of state-of-the-art classification algorithms applied to lung tissue categorization. Digit Imaging 23:18–30
Zavaletta VA, Bartholmai BJ, Robb RA (2007) High resolution multidetector CT-aided tissue analysis and quantification of lung fibrosis. Acad Radiol 14:772–787
Iwasawa T, Asakura A, Sakai F, Kanauchi T, Gotoh T, Ogura T, Yazawa T, Nishimura J, Inoue T (2009) Assessment of prognosis of patients with idiopathic pulmonary fibrosis by computer-aided analysis of CT images. J Thorac Imaging 24:216–222
Arzhaeva Y, Prokop M, Murphy K, van Rikxoort EM, de Jong PA, Gietema HA, Viergever MA, van Ginneken B (2010) Automated estimation of progression of interstitial lung disease in CT images. Med Phys 37:63–73
Best AC, Meng J, Lynch AM, Bozic CM, Miller D, Grunwald GK, Lynch DA (2008) Idiopathic pulmonary fibrosis: physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology 246:935–940
Mahler DA, Weinberg DH, Wells CK, Feinstein AR (1984) The measurement of dyspnea: contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest 85:751–758
Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, Elashoff RM, Furst DE, Vasunilashorn S, McNitt-Gray MF, Brown MS, Roth MD, Tashkin DP, Scleroderma Lung Study Research Group (2008) High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 134:358–367
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Khanna D, Yan X, Tashkin DP, Furst DE, Elashoff R, Roth MD, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M, Clements PJ, Scleroderma Lung Study Group (2007) Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum 56(5):1676–1684
Wells AU, Behr J, Silver R (2008) Outcome measures in the lung. Rheumatology 47(Suppl 5):v48–v50
Prentice RL (1989) Surrogate endpoints in clinical trials: definitions and operational criteria. Stat Med 8:431–440
Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H (2000) The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1:49–67
Altar CA, Amakye D, Bounos D, Bloom J, Clack G, Dean R, Devanarayan V, Fu D, Furlong S, Hinman L, Girman C, Lathia C, Lesko L, Madani S, Mayne J, Meyer J, Raunig D, Sager P, Williams SA, Wong P, Zerba K (2008) A prototypical process for creating evidentiary standards for biomarkers and diagnostics. Clin Pharmacol Ther 83:368–371
Acknowledgements
Supported by Public Health Service grants from the National Heart, Lung, and Blood Institute and the National Institute of Arthritis and Musculoskeletal and Skin Diseases; cyclophosphamide (Cytoxan) was supplied by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix

Bland-Altman plot of quantitative lung fibrosis (QLF) score that was calculated repeatedly using a random sampling technique from 4 by 4 grid. a Mean difference was −0.008% (±0.36). The two dashed lines are 0.72% and −0.72%. For the outlying point where the difference was close to −2%, the patient had severe disease, with QLF score on the 1st run of 76.68% and 74.71% on the 2nd run. b Mean difference was 0.008% (±0.072). The two dashed lines are 0.14% and −0.14%. In Bland Altman plots, two boundaries are ±0.72% from the most severe zones and ±0.14% from the whole lung, indicating the small variation from a sampling technique from the 4 by 4 grid in the classifier model. Differences in QLF score greater than potential variation due to random sampling were demonstrated in 93% subjects (77/83) in the most severe zone at baseline and in 96% subjects (80/83) in the whole lung.
Rights and permissions
About this article
Cite this article
Kim, H.J., Brown, M.S., Elashoff, R. et al. Quantitative texture-based assessment of one-year changes in fibrotic reticular patterns on HRCT in scleroderma lung disease treated with oral cyclophosphamide. Eur Radiol 21, 2455–2465 (2011). https://doi.org/10.1007/s00330-011-2223-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2223-2