Abstract
Summary
We explored the cardiac safety of the osteoporosis treatment strontium ranelate in the UK Clinical Practice Research Datalink. While known cardiovascular risk factors like obesity and smoking were associated with increased cardiac risk, use of strontium ranelate was not associated with any increase in myocardial infarction or cardiovascular death.
Introduction
It has been suggested that strontium ranelate may increase risk for cardiac events in postmenopausal osteoporosis. We set out to explore the cardiac safety of strontium ranelate in the Clinical Practice Research Datalink (CPRD) and linked datasets.
Methods
We performed a nested case–control study. Primary outcomes were first definite myocardial infarction, hospitalisation with myocardial infarction, and cardiovascular death. Cases and matched controls were nested in a cohort of women treated for osteoporosis. The association with exposure to strontium ranelate was analysed by multivariate conditional logistic regression.
Results
Of the 112,445 women with treated postmenopausal osteoporosis, 6,487 received strontium ranelate. Annual incidence rates for first definite myocardial infarction (1,352 cases), myocardial infarction with hospitalisation (1,465 cases), and cardiovascular death (3,619 cases) were 3.24, 6.13, and 14.66 per 1,000 patient-years, respectively. Obesity, smoking, and cardiovascular treatments were associated with significant increases in risk for cardiac events. Current or past use of strontium ranelate was not associated with increased risk for first definite myocardial infarction (odds ratio [OR] 1.05, 95 % confidence interval [CI] 0.68–1.61 and OR 1.12, 95 % CI 0.79–1.58, respectively), hospitalisation with myocardial infarction (OR 0.84, 95 % CI 0.54–1.30 and OR 1.17, 95 % CI 0.83–1.66), or cardiovascular death (OR 0.96, 95 % CI 0.76–1.21 and OR 1.16, 95 % CI 0.94–1.43) versus patients who had never used strontium ranelate.
Conclusions
Analysis in the CPRD did not find evidence for a higher risk for cardiac events associated with the use of strontium ranelate in postmenopausal osteoporosis.
Similar content being viewed by others
Introduction
Strontium ranelate has been in clinical use since 2004 for the management of postmenopausal osteoporosis, for which it reduces the risk of vertebral and nonvertebral fracture [1, 2]. More recently, it has also been approved for the management of osteoporosis in men at increased risk of fracture [3], and tested in patients with osteoarthritis [4, 5]. A large number of phase 2 and 3 clinical trials have been carried out, including more than 8,000 patients on strontium ranelate with nearly 36,000 patient-years of exposure [6]. A recent pooled analysis in 7,572 postmenopausal women (3,803 strontium ranelate and 3,769 placebo) indicated an increased risk for myocardial infarction (MI) with strontium ranelate, with estimated annual incidences of 5.7 cases per 1,000 patient-years versus 3.6 cases per 1,000 patient-years with placebo [6]. This translates into an odds ratio (OR) for MI of 1.60 (95 % confidence interval [CI], 1.07–2.38) for strontium ranelate versus placebo (incidences of 1.7 % versus 1.1 %, respectively) [6]. Among the cases of MI, fatal events were less frequent with strontium ranelate (15.6 %) than with placebo (22.5 %). In order to reduce the risk in treated patients in routine clinical practice, new contraindications have been proposed for strontium ranelate in patients with a history of cardiovascular disease (history of ischaemic heart disease, peripheral artery disease, and cerebrovascular disease, and uncontrolled hypertension) [7]. Exclusion of patients with these contraindications from the pooled analysis mitigated the risk for MI (OR, 0.99; 95 % CI, 0.48–2.04; data on file).
There has been no suggestion of excessive cardiac events in postmarketing surveillance data for strontium ranelate covering more than 3.4 million patient-years of treatment from September 2004 to February 2013. There have been 16 cases of MI spontaneously reported over the 96-month period of monitoring, i.e. a rate of 0.5 cases per 100,000 patient-years [6]. Similarly, an observational prospective cohort study including 12,076 patients on strontium ranelate with 80 % adherence over 2 years did not support increased incidence of cardiac events over the 32.0 ± 9.7 months of follow-up; there were 33 cases of MI in the cohort (1.3 per 1,000 patient-years) [6, 8].
In this paper, we describe a nested case–control study performed within the UK Clinical Practice Research Datalink (CPRD) apparatus to further explore the risk for ischaemic cardiac events associated with the use of strontium ranelate in routine clinical practice in women with postmenopausal osteoporosis.
Methods
Study population
The main data source for this nested case–control study was the CPRD, which comprises anonymous electronic medical records from primary care in the UK and covers about 8 % of the population. Contributing CPRD physicians come from some 640 practices throughout the UK, which must meet specific up-to-standard (UTS) reporting requirements defined by the CPRD. The accuracy and completeness of the CPRD dataset has been confirmed [9, 10], as has the predictive value of the database for cardiac events, including MI [11, 12]. The positive predictive value of the CPRD to detect acute MI, for example, is 93 % (95 % CI, 90–96 %), i.e. the proportion of cases with acute MI codes that are confirmed as true cases of acute MI [12]. In our study, according to the outcome explored, the CPRD data were linked to the Hospital Episode Statistics (HES) and the Office of National Statistics (ONS) databases to obtain additional information on hospitalisations and fatalities, respectively. The study protocol was approved by the Independent Scientific Advisory Committee of the Medicines and Healthcare Products Regulatory Agency (MHRA).
We identified all male and female patients who had received a prescription for osteoporosis treatment or a medical record of primary osteoporosis between 1 January 2002 and 30 April 2012. The cohort entry date was fixed as the date of the first prescription of osteoporosis treatment during the study period. Patients were excluded if they had had a prescription for an osteoporosis treatment in the previous year or had received a prescription for bisphosphonate for indications other than osteoporosis (e.g., Paget’s disease, hypercalcaemia, breast cancer, or myeloma). Patients could also be excluded if they came from a practice with less than 1 year of UTS CPRD data at their cohort entry date. From this population, we then excluded successively patients who had never received a treatment for their osteoporosis, and then all male patients, to reach a population of women with treated osteoporosis.
The follow-up period extended from the cohort entry date to the date of the last data collection from the practice, the date of transfer if the patient left the practice, or the date of death.
Outcomes and selection of controls
The primary outcomes of our nested case–control study were first definite MI (fatal or nonfatal), hospitalisation with MI (fatal or nonfatal, first or subsequent), and cardiovascular death occurring after the cohort entry date. The index date for cases was defined as the date of event. Cases of MI were qualified as definite [13] if there was a CPRD record of MI, and the patient either (1) died within 30 days, or (2) was initiated on relevant treatment (e.g., statins, nitrates, and/or beta-blockers), and had other supporting evidence (e.g., location of infarct, coronary artery revascularisation, and/or elevated cardiac enzymes) within 2 months of the MI. Analyses on first definite MI excluded patients with previous MI. Cases of hospitalisation with MI were identified in the HES dataset in patients eligible for linkage, which ensured detection of cases not otherwise apparent in the GP record. Analyses of cases of hospitalisation with MI did not exclude patients with previous MI. Cases of cardiovascular death were identified in the ONS death dataset in patients eligible for linkage. This dataset provides information on cause and date of death, which may be missing in the general practice-based CPRD.
Three case–control analyses were performed successively. Thus, for each case of first definite MI, hospitalisation with MI, or cardiovascular death, six to ten controls without the event prior to the index date were randomly selected by risk set sampling within the treated osteoporosis cohort. The index date attributed to controls was the same as in the corresponding case. Cases and controls were matched on year of birth (exact matching criterion), calendar date of event, and prior osteoporosis treatment duration ±1 year (i.e. time since first prescription of any osteoporosis treatment as a proxy for disease severity).
Treatment exposure
Treatment exposure was calculated on the basis of the records of prescriptions issued by general practitioners according to routine clinical practice in the UK [14]. Exposure to strontium ranelate before the index date was compared between cases and controls. Similar analyses were performed in patients with exposure to alendronate as a reference treatment in osteoporosis. Current use was defined as having an ongoing prescription for the treatment at the index date (or within the previous month). Past use was defined as cessation of the treatment more than 1 month prior to the index date. Patients who had never had a prescription for the treatment before the index date were used as a reference group.
Statistical methods
The characteristics of the patients are presented as descriptive statistics at cohort entry date for women with treated osteoporosis, and at date of treatment initiation for women receiving strontium ranelate or alendronate. For each outcome, the annual incidence rate (IR) per 1,000 patient-years was estimated in the cohort of women with treated osteoporosis with the 95 % confidence interval (CI) based on a Poisson or normal approximation. The comparisons between cases and controls were based on a multivariate conditional logistic regression. We estimated the effect of region, prior UTS follow-up duration, socioeconomic status, obesity (body mass index ≥30 kg/m2 or diagnosis), smoking (yes/no), antidiabetic treatments, statins/fibrates, antihypertensive treatments (beta-blockers, calcium channel blockers, renin–angiotensin system inhibitors, and/or diuretics), platelet inhibitors (including aspirin), nitrates, hormone replacement therapy, calcium and vitamin D supplementation, other osteoporosis treatment, and history of MI. Patients with current use or past use of strontium ranelate were compared with patients who had never used strontium ranelate. The odds ratios associated with the considered treatment effect in the unadjusted and fully adjusted models were provided as well as their accuracy (two-sided 95 % CI). Fully adjusted analyses were based on a backward selection of all factors significant in the univariate analysis for the outcome in question (20 % threshold). The same methodology was used to compare patients with current use or past use of alendronate with patients who had never used alendronate. All statistical analyses were conducted using SAS® software version 9.2.
Results
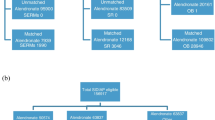
The selection of patients for this nested case–control study is presented in Fig. 1. Out of over 220,000 patients with a diagnosis of primary osteoporosis and/or a prescription for an osteoporosis treatment between 1 January 2002 and 30 April 2012 in the CPRD, we identified a cohort of 112,445 women with treated osteoporosis. At entry to the cohort, patients were aged 71.8 ± 12.7 years; they had BMI of 25.5 ± 5.3 kg/m2, and 15 % were classified as obese (Table 1). The rate of smoking was 20 %. Time since diagnosis of osteoporosis was 21.5 ± 49.2 months. About half were receiving cardiovascular treatments such as antihypertensives or platelet inhibitors. Two thirds of the patients were receiving calcium and vitamin D supplementation.
During the follow-up period, 6,487 patients received strontium ranelate and 94,654 received alendronate. The mean cumulative exposure for strontium ranelate was 12.8 ± 16.4 months (with a maximum of 87 months), while that for alendronate was 25.4 ± 26.0 months. The patients receiving strontium ranelate were older than the general cohort of women with treated osteoporosis and had a longer time since diagnosis; they were also more likely to be receiving concomitant supplementation with calcium and vitamin D (Table 1).
There were 1,352 cases of first definite MI in the cohort of women with treated osteoporosis (IR 3.24 per 1,000 patient-years; 95 % CI, 3.07–3.41). Of these, 16 cases were excluded from the analysis due to failure to identify six to ten matching controls, leaving 1,336 cases and 13,330 matching controls. The mean age of the cases and controls was 79.5 years with mean osteoporosis treatment duration of 39 months (Table 2). Less than 5 % of cases and controls had been previously exposed to strontium ranelate, while about 80 % had been exposed to alendronate. The mean cumulative prior exposure to strontium ranelate was 10.9 ± 13.9 (64 cases, with a maximum duration of 57 months) and 10.7 ± 13.6 months (615 controls), and the mean cumulative prior exposure of alendronate was 19.6 ± 21.6 (1,060 cases) and 21.0 ± 21.5 months (10,494 controls). Results for first definite MI are presented in Table 2. In the unadjusted analysis, as would be expected, obesity, smoking, and use of antidiabetics, statins/fibrates, antihypertensives, and platelet inhibitors were associated with significant increases in risk for first definite MI. Current or past use of strontium ranelate was not associated with an increase in risk for first definite MI compared with patients who had never received strontium ranelate (adjusted OR 1.05, 95 % CI 0.68–1.61 and OR 1.12, 95 % CI 0.79–1.58, respectively). Similar results were found for current or past use of alendronate (adjusted OR 0.98, 95 % CI 0.83–1.15 and OR 1.09, 95 % CI 0.92–1.30).
There were 1,465 cases of hospitalisation with MI in the cohort of women with treated osteoporosis (IR 6.13 per 1,000 patient-years, 95 % CI 5.81–6.44). Of these, 32 cases were excluded from the analysis (matching failure), and results for hospitalisation for MI in 1,433 cases and 14,261 matched controls are presented in Table 3. These patients were aged 81.1 years, and <5 % had previously received strontium ranelate (67 cases and 613 controls) and about 80 % alendronate (1,130 cases and 11,424 controls). The durations of prior osteoporosis treatment exposure were very similar to those reported for the analysis of first definite MI. Obesity, smoking, and the use of antidiabetics, statins and fibrates, antihypertensives, and platelet inhibitors were all found to increase the risk for hospitalisation with MI. There was a particularly strong association for previous hospitalisation with MI, which increased risk for recurrent hospitalisation with MI by almost four times (OR 3.79, 95 % CI 3.16–4.55). Current or past use of strontium ranelate was not associated with a significant increase in risk for hospitalisation with MI (adjusted OR 0.84, 95 % CI 0.54–1.30 and OR 1.17, 95 % CI 0.83–1.66). Patients with current use of alendronate were at borderline lower risk for hospitalisation with MI than patients who had never used alendronate (adjusted OR 0.85, 95 % CI 0.73–0.99), though the effect was not found for patients with past use of alendronate (adjusted OR 1.17, 95 % CI 0.99–1.37).
There were 3,619 cardiovascular deaths in the cohort of women with treated osteoporosis (IR 14.66 per 1,000 patient-years, 95 % CI 14.18–15.14). Of these, 103 cases were excluded from the analysis (matching failure), leaving 3,516 cases, which were compared with 34,982 matched controls (Table 4). Cases and controls were aged 83.9 years. The durations of cumulative prior exposure to strontium ranelate (195 cases and 1,689 controls) and alendronate (2,732 cases and 27,573 controls) were similar to that described for the analysis of first definite MI. Obesity, smoking, and use of antidiabetics, statins/fibrates, antihypertensives, and platelet inhibitors were associated with higher risk for cardiovascular death. Current or past use of strontium ranelate was not associated with a significant increase in risk for cardiovascular death versus patients who had never received the treatment (adjusted OR 0.96, 95 % CI 0.76–1.21, and OR 1.16, 95 % CI 0.94–1.43). Current use of alendronate was associated with a reduction in the risk for cardiovascular death versus patients who had never used alendronate (adjusted OR 0.80, 95 % CI 0.72–0.88), while past use was associated with a borderline increase in risk for cardiovascular death versus patients who had never used alendronate (adjusted OR 1.11, 95 % CI 1.01–1.23).
Discussion
The purpose of this retrospective observational study using the UK CPRD and linked datasets was to evaluate whether the use of strontium ranelate in routine clinical practice is associated with increased risk for ischaemic cardiac events. This nested case–control study was based on a cohort encompassing over 110,000 women treated for osteoporosis, mostly with alendronate. A small proportion was receiving strontium ranelate.
In our study, current use of strontium ranelate in patients with postmenopausal osteoporosis was not associated with increased risk for first definite MI versus patients who had never received the treatment. Similar results were found for hospitalisation with MI and cardiovascular death, and for patients who had used the treatment in the past. Our results also suggest that current use of alendronate could have a cardioprotective effect. This is not the first such finding for alendronate [15], but the underlying reasons remain unclear, and the use of a retrospective, observational, case–control study design, as well as the borderline significance of the result precludes firm conclusions on this point until further research is performed.
The mean duration of prior exposure to strontium ranelate was around 1 year for cases and controls. Although longer-term exposure is not available in CPRD, these data reflect the real-life pattern of strontium ranelate use from clinical practice in the UK.
The robustness of the analysis is demonstrated by the consistency of our observations over the three outcomes considered. A number of sensitivity analyses have been performed using various definitions of exposure. These led to consistent results (data not shown). Moreover, the observation of the effects of established cardiovascular risk factors, e.g., smoking, obesity, and previous hospitalisation with MI, on subsequent cardiac events [16] supports the validity of our study. Also, even though there were many risk and confounding factors included in the multivariate analysis, there was little difference between the adjusted and unadjusted results for the treatment effect.
There are a number of limitations to our study. Several possible confounders are not recorded in the CPRD such as severity of osteoporosis, bone mineral density, menopause, physical activity, and family history of ischaemic cardiac events. However, the nested case–control design handles the heterogeneity of the population (by matching cases with controls using the most important potential confounders and adjusting the analyses on the remaining risk and confounding factors). There is a potential for channelling bias due to confounding by severity of osteoporosis or possible links between osteoporosis and cardiovascular disease [17]. This may limit direct comparison between strontium ranelate and alendronate because strontium ranelate is generally given only when alendronate is not acceptably effective [14]. Finally, adherence to treatment may be overestimated since drug prescribing does not necessarily equate with drug use, though sensitivity analyses using various definitions of drug exposure gave similar results.
Further caveats to our study include the absence of a control group without osteoporosis and the use of propensity matching for our cases and controls. This leaves open the potential for confounding by indication, with regard to treatment using alendronate or strontium ranelate, following diagnosis of osteoporosis. The reduced risk of MI among predominantly alendronate users might represent just such a selection artefact. Finally, the pattern of osteoporosis prescribing in the UK [14] left the selected cohort of women treated for osteoporosis, as predominantly receiving alendronate (84 %). Only 6 % of the treated women received strontium ranelate; and only 14 % had never used either strontium ranelate or alendronate. Thus, the ability to contrast strontium ranelate treatment with the cardiovascular experience of women in the UK population as a whole or with women using osteoporosis treatment other than alendronate was limited. The study sample utilised was necessary to maximise the prevalence of the exposure of interest (strontium ranelate), but future research could include a more traditional retrospective cohort study in patients treated with strontium ranelate, alendronate, osteoporosis with other treatments, and women selected from the CPRD as a whole.
Nonetheless, much effort was made to reduce bias in this retrospective observational study. The sensitivity of the algorithm for first definite MI has been tested and confirmed [13], and the reliability of the identification of cardiac outcomes is further reinforced by the use of hard endpoints and linkage to ONS/HES data. The case–control analysis was nested in a cohort of women who were all treated for osteoporosis to reduce selection bias due to potential heterogeneity between patients. The design also accounts for the two main confounders related to clinical use of strontium ranelate in the UK [14]: calendar date, because strontium ranelate has been available for a short time relative to other osteoporosis treatments, and disease duration, because strontium ranelate is recommended second or third line, while alendronate, for example, is usually prescribed first line. This is clear from the patient characteristics, which show that patients treated with strontium ranelate were older than the patients with osteoporosis treated with other agents and had a longer time since diagnosis.
Our study highlights a substantial relative risk for cardiac events associated with previous hospitalisation with MI in patients with treated postmenopausal osteoporosis. The current labeled indication for drug administration according to the European Medicines Agency approval for strontium ranelate includes a contraindication in patients at risk for cardiovascular events (history of ischaemic heart disease, peripheral artery disease, cerebrovascular disease, and uncontrolled hypertension) [7]. Our analysis indicates that the risk for cardiac events is increased in patients with these contraindications. Indeed, in the case–control analysis of hospitalisation with MI, 12 % of the cases and 4 % of the controls had had a history of previous hospitalisation with MI before index date. Similar elevated risks were found for history of ischaemic heart disease (71 % in cases versus 24 % in controls), peripheral artery disease (18 % in cases versus 7 % in controls), and cerebrovascular disease (23 % in cases versus 15 % in controls). In line with this, exclusion of patients with the contraindications from the pooled analyses of the randomised-controlled trials with strontium ranelate completely mitigated the risk for MI (data on file). The new contraindications for strontium ranelate are therefore expected to reduce any potential cardiovascular risk associated with use of this treatment.
Conclusion
The results of this nested case–control study in the CPRD indicate no evidence for a higher risk of MI or cardiovascular death associated with the use of strontium ranelate in women treated for osteoporosis compared with non-use of this agent in routine medical practice in the UK.
References
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Reginster J-Y, Felsenberg D, Boonen S et al (2008) Effects of long-term strontium ranelate treatment on the risk of nonvertebral and vertebral fractures in postmenopausal osteoporosis: results of a five-year, randomized, placebo-controlled trial. Arthritis Rheum 58:1687–1695
Kaufman JM, Audran M, Bianchi G et al (2013) Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab 98:592–601
Reginster JY, Badurski J, Bellamy N et al (2013) Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis 72:179–186
Cooper C, Reginster J-Y, Chapurlat R et al (2012) Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of a randomised double-blind, placebo-controlled trial. Curr Med Res Opin 28:231–239
European Medicines Agency (2013) PSUR assessment report—strontium ranelate. www.ema.europa.eu. Accessed 27 Aug 2013
European Medicines Agency (2006) Summary of product characteristics. Protelos. European Medicines Agency. http://www.ema.europa.eu. Accessed 19 Sept 2013
Audran M, Jakob FJ, Palacios S et al (2013) A large prospective European cohort study of patients treated with strontium ranelate and followed up over 3 years. Rheumatol Int 33:2231–2239
Khan NF, Harrison SE, Rose PW (2010) Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 60:e128–e136
Herrett E, Thomas SL, Schoonen WM et al (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69:4–14
Varas-Lorenzo C, Garcia-Rodriguez LA, Perez-Gutthann S et al (2000) Hormone replacement therapy and incidence of acute myocardial infarction. A population-based nested case–control study. Circulation 101:2572–2578
Hammad TA, McAdams MA, Feight A et al (2008) Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiol Drug Saf 17:1197–1201
Mulnier HE, Seaman HE, Raleigh VS et al (2008) Risk of myocardial infarction in men and women with type 2 diabetes in the UK: a cohort study using the General Practice Research Database. Diabetologia 51:1639–1645
National Institute for Health and Clinical Excellence (2011) Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. NICE technology appraisal guidance TA160. National Institute for Health and Clinical Excellence. www.nice.org.uk/TA160. Accessed 29 Aug 2013
Kang JH, Keller JJ, Lin HC (2013) Bisphosphonates reduced the risk of acute myocardial infarction: a 2-year follow-up study. Osteoporos Int 24:271–277
Graham I, Atar D, Borch-Johnsen K et al (2007) European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 28:2375–2414
Lampropoulos CE, Papaioannou I, D’Cruz DP (2012) Osteoporosis—a risk factor for cardiovascular disease? Nat Rev Rheumatol 8:587–598
Acknowledgments
The interpretation and conclusions contained in this report are those of the authors alone. This study was funded by Servier. Study data were obtained from the CPRD under license from the UK MHRA to the Acceptability Data and Pharmacoepidemiology Department of Servier. The authors would like to thank Karine Marinier and Nicolas Deltour (Servier) for help with study design and conduct and statistical analysis. KF is an NIHR Senior Investigator supported by the NIHR Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital.
Conflicts of interest
All authors have disclosed receiving fees, honoraria, and research grants from Servier.
Author information
Authors and Affiliations
Corresponding author
Additional information
A related article can be found at DOI 10.1007/s00198-013-2469-4; a related editorial at DOI 10.1007/s00198-013-2583-3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Cooper, C., Fox, K.M. & Borer, J.S. Ischaemic cardiac events and use of strontium ranelate in postmenopausal osteoporosis: a nested case–control study in the CPRD. Osteoporos Int 25, 737–745 (2014). https://doi.org/10.1007/s00198-013-2582-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2582-4