Summary
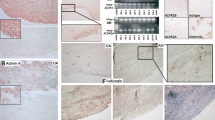
In view of the important role of interstitial collagenase in the pathogenesis of rheumatoid arthritis (RA), we studied the expression of fibroblast-type collagenase in rheumatoid synovium and searched for its potential transcription factors, namely the oncoprotein c-fos and the early-growth-response gene-1 (egr-1), an inducible zinc-finger encoding gene. Elevated levels of RNA sequences complimentary to c-fos and egr-1 cDNA probes could be detected in cytoplasmic extracts of collagenase-expressing synovial fibroblast-like cells when compared to equivalent RNA amounts isolated from control fibroblasts. Utilizing immunocytochemistry, immunoreactivity for c-fos oncoprotein was found in 13 of 19 joint specimens obtained from patients with active RA. These oncoprotein data were positively correlated to the collagenase expression in the same specimens. Moreover, immunohistochemical analysis confirmed the localization of both oncoprotein c-fos and fibroblast-type collagenase within synovial fibroblast-like cells attached to bone erosions.
Similar content being viewed by others
References
Krane SM, Conca W, Stephenson ML, Amento EP, Goldring MB (1990) Mechanisms of matrix degradation in rheumatoid arthritis. Ann NY Acad Sci 580:340–354
Stricklin GP, Hibbs MS (1988) Biochemistry and physiology of mammalian collagenases. In: Nimni ME (ed) Collagen, vol 1. CRC, Boca Raton, Fla, pp 187–205
Birkedal-Hansen H (1988) From tadpole collagenase to a family of matrix metalloproteinases. J Oral Pathol 17:445–451
Birkedal-Hansen B, Moore WGI, Taylor RE, Bhown AS, Birkedal-Hansen H (1988) Monoclonal antibodies to human fibroblast procollagenase. Inhibition of enzymatic activity, affinity purification of the enzyme, and evidence for clustering of epitopes in the NH2-terminal end of the activated enzyme. Bochemistry 27:6751–6758
Eeckhout Y, Vaes G (1977) Further studies on the activation of procollagenase, the latent precursor of bone collagenase: Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J 166:21–31
Nagase H, Cawston TE, De Silva M, Barrett AJ (1982) Identification of plasma kallikrein as an activator of latent collagenase in rheumatoid synovial fluid. Biochim Biophys Acta 702:133–142
Stricklin GP, Jeffrey JJ, Roswit WT, Eisen AZ (1983) Human skin fibroblast procollagenase: Mechanisms of activation by organomercurials and trypsin. Biochemistry 22:61–68
Suzuki K, Nagase H, Ito A, Enghild JJ, Salvesen G (1990) The role of matrix metalloproteinase 3 in the stepwise activation of human rheumatoid synovial procollagenase. Biol Chem Hopp Seyler 371 [Suppl]:305–310
Matrisian LM, Hogan BLM (1990) Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol 24:219–259
Schönthal A, Herrlich P, Rahmsdorf HJ, Ponta H (1988) Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell 54:325–344
Sukhatme VP, Kartha S, Toback FG, Taub R, Hoover RG, Tsai-Morris C (1987) A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res 1:343–355
Sukhatme VP, Cao X, Chang LC, Tsai-Morris C, Stamenkovich D, Ferreira PCP, Cohen DR, Edwards SA, Shows TB, Curran T, Le Beau MM, Adamson ED (1988) A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell 53:34–43
Tsai-Morris C, Cao X, Sukhatme VP (1988) 5′ flanking sequence and genomic structure of Egr-1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res 16:8835–8846
Suggs SV, Katzowitz JL, Tsai-Morris C, Sukhatme VP (1990) cDNA sequence of the human cellular early growth response gene Egr-1. Nucleic Acids Res 18:4283
Janssen-Timmen U, Lemaire P, Mattéi MG, Revelant O, Charnay P (1989) Structure, chromosome mapping and regulation of the mouse zinc-finger gene Krox-24; evidence for a common regulatory pathway for immediate-early serum-response genes. Gene 80:325–336
Trabandt A, Aicher WK, Gay RE, Sukhatme VP, Nilson-Hamilton M, Hamilton RT, McGhee JR, Fassbender HG, Gay S (1990) Expression of the collagenolytic and ras-induced cysteine proteinase cathepsin L and proliferation-associated oncogenes in synovial cells of MRL/1 mice and patients with rheumatoid arthritis. Matrix 10:349–361
Miller AD, Curran T, Verma IM (1984) c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell 36:51–60
White BA, Bancroft FC (1982) Cytoplasmic dot hybridization. J Biol Chem 257:8569–8572
Feinberg A, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Overall CM, Sodek J (1990) Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. J Biol Chem 265:21141–21151
Sukhatme VP (1990) Early transcriptional events in cell growth: the egr family. J Am Soc Nephrol 1:859–866
Kerr LD, Holt JT, Matrisian LM (1988) Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science 242:1424–1427
Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH (1989) Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109:877–889
Tremble P, Werb Z (1990) Regulation of metalloproteinase gene expression by the fibronectin receptor involves a TPA-responsive element and c-fos. J Cell Biol 111:263a
Vance BA, Dowalski CG, Brinckerhoff CE (1989) Heat shock of rabbit synovial fibroblasts increases expression of mRNAs for two metalloproteinase, collagenase and stromelysin. J Cell Biol 108:2037–2043
Ransone LJ, Verma IM (1990) Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol 6:539–557
Whitham SE, Murphy G, Angel P, Rahmsdorf H-J, Smith BJ, Lyons A, Harris TFJ, Reynolds JJ, Herrlich P, Docherty AJP (1986) Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J 240:913–916
Gay S, Gay RE (1989) Cellular basis and oncogene expression of rheumatoid joint destruction. Rheumatol Int 9:105–113
Fassbender HG (1983) Histomorphologic basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res 3:141–155
Tanaka A, O'Sullivan FX, Koopman WJ, Gay S (1988) Etiopathogenesis of the rheumatoid arthritis-like disease in MRL/1 mice: II. Ultrastructural basis of joint destruction. J Rheumatol 15:10–16
Woolley DE, Crossley MJ, Evanson JM (1977) Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum 20:1231–1239
Trabandt A, Gay RE, Birkedal-Hansen H, Gay S (1992) Expression of collagenase and potential transcriptional factors in the MRL/1 mouse arthropathy. Semin Arthritis Rheum 21:246–251
Milbrandt J (1987) A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science 238:797–799
Christy BA, Lau LF, Nathans D (1988) A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with “zinc-finger” sequences. Proc Natl Acad Sci USA 85:7857–7861
Lemaire P, Revelant O, Bravo R, Charnay P (1988) Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci USA 85:4691–4695
Lim RW, Varnum BC, Herschman HR (1987) Cloning of tetradecanoyl phorbol ester-induced “primary response” sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene 1:263–270
Greenberg ME, Ziff EB (1984) Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311:433–438
Thoenen H, Barde Y-A, Edgar D (1981) The role of nerve growth factor (NGF) and related factors the survival of peripheral neurons. In: Martin JB, Reichlin S, Bick KL (eds) Neurosecretion and brain peptides. Raven, New York, pp 263–273
Nishikura K, Murray JM (1987) Antisense RNA of protooncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol 7:639–649
Riabowol KT, Vosatka RJ, Ziff EB, Lamb NJ, Feramisco JR (1988) Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol 8:1670–1676
McMahon AP, Champion JE, McMahon JA, Sukhatme VP (1990) Developmental expression of the putative transcription factor Egr-1 suggests that Egr-1 and c-fos are coregulated in some tissues. Development 108:281–287
Sandberg M, Vuorio T, Hirvonen H, Alitalo K, Vuorio E (1988) Enhanced expression of TGF-β and c-fos mRNAs in the growth plates of developing human long bones. Development 102:461–470
Closs EI, Murray AB, Schmidt J, Schön A, Erfle V, Strauss PG (1990) c-fos expression precedes osteogenic differentiation of cartilage cells in vitro. J Cell Biol 111:1313–1323
Wright JJ, Gunter KC, Mitsuya H, Irving SG, Kelly K, Siebenlist U (1990) Expression of a zinc finger gene in HTLV-I and HTLV-II transformed cells. Science 248:588–591
Aicher WK, Gay RE, Stransky G, Trabandt A, Bridges SL Jr, Schroeder HW, Koopman WJ, Siebenlist U, Gay S (1991) Detection of Z-225 message in a cDNA library and synovial lining cells of patients with rheumatoid arthritis (RA). Arthritis Rheum 34[S]:44
Stransky G, Moreland LW, Gay RE, Gay S (1990) Virus-like particles (VLP) in synovial fluids from patients with rheumatoid arthritis (RA). Arthritis Rheum 33[S]:143
Stransky G, Aicher WK, Gay RE, Gay S (1991) Characterization of virus-like particles (VLP) derived from patients with rheumatoid arthritis (RA). Arthritis Rheum 34[S]:177
Trabandt A, Gay RE, Stransky G, Aicher WK, Gay S (1991) Cellular and molecular basis of rheumatoid joint destruction. In: Talal N (ed) Molecular autoimmunity. Academic Press, London, pp 233–245
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trabandt, A., Aicher, W.K., Gay, R.E. et al. Spontaneous expression of immediately-early response genes c-fos and egr-1 in collagenase-producing rheumatoid synovial fibroblasts. Rheumatol Int 12, 53–59 (1992). https://doi.org/10.1007/BF00300977
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00300977