Article Text
Abstract
Objectives To investigate the ability of high-sensitivity C-reactive protein (hsCRP) and S100A12 to serve as predictive biomarkers of successful drug withdrawal in children with clinical remission of juvenile idiopathic arthritis (JIA).
Methods This multicentre trial (PREVENT-JIA) enrolled 119 patients with JIA in clinical remission, and 100 patients reached the intervention phase in which the decision whether to continue or stop treatment was based on S100A12 and hsCRP levels. Patients were monitored for 12 months after stopping medication for flares of disease. Results were compared with withdrawal of therapy without biomarker-based stratification in patients from the German Biologika in der Kinderrheumatologie (BiKeR) pharmacovigilance registry.
Results In the PREVENT-JIA group, 49 patients had a flare, and 45% of patients stopping medication showed flares within the following 12 months. All patients (n=8) continuing therapy due to permanently elevated S100A12/hsCRP at more than one visit flared during the observation phase. In the BiKeR control group, the total flare rate was 62%, with 60% flaring after stopping medication. The primary outcome, time from therapy withdrawal to first flare (cumulative flare rate after therapy withdrawal), showed a significant difference in favour of the PREVENT-JIA group (p=0.046; HR 0.62, 95% CI 0.38 to 0.99). As additional finding, patients in the PREVENT-JIA trial stopped therapy significantly earlier.
Conclusion Biomarker-guided strategies of therapy withdrawal are feasible in clinical practice. This study demonstrates that using predictive markers of subclinical inflammation is a promising tool in the decision-making process of therapy withdrawal, which translates into direct benefit for patients.
Trial registration number ISRCTN69963079.
- Juvenile idiopathic arthritis (JIA)
- biomarker
- therapy withdrawal
- immunological remission
- relapse prevention
- subclinical inflammation
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
- Juvenile idiopathic arthritis (JIA)
- biomarker
- therapy withdrawal
- immunological remission
- relapse prevention
- subclinical inflammation
Key messages
What is already known about this subject?
Decision-making for therapy withdrawal in clinical remission of juvenile idiopathic arthritis remains challenging.
What does this study add?
S100A12/high-sensitivity C-reactive protein as markers of subclinical inflammation may help to evaluate the risk of flares and guide treatment decision.
A risk-adapted strategy reduces both the number of flares in remission and the cumulative drug exposure.
How might this impact on clinical practice or future developments?
Biomarker-guided strategies of therapy withdrawal are feasible in clinical practice.
A stratified approach to therapy withdrawal is superior to decision-making without predefined strategy.
Introduction
Juvenile idiopathic arthritis (JIA) is a potentially disabling chronic disease of childhood.1 Although it is now treatable, JIA frequently takes a remitting disease course. Patients require immunosuppression for years, and the long-term outcome is not easy to predict.2 Fewer than half of the patients with JIA managed with contemporary treatments achieve remission off medication within 5 years of diagnosis.3 In this context clinical remission is defined as inactive disease of at least 6 months continuously. While this may argue for prolonged maintenance treatment, any anti-inflammatory medication therapy is associated with cost, potential side effects and administration of drugs can be stressful for the patients and the family.4–6 However, there is little guidance in which context medication can be withdrawn safely, while avoiding disease relapses and potentially permanent damage in a child with JIA.7 Indeed, current treatment guidelines are devoid of considering predictive molecular markers to identify patients with JIA who can tolerate medication withdrawal.
A controlled trial on methotrexate (MTX) withdrawal demonstrated that the flare risk is independent from the duration of therapy after achieving remission.8 Post-hoc analyses revealed that patients with both low levels of S100A12 and low levels of high-sensitivity C-reactive protein (hsCRP) have a similar flare risk off medication compared with patients with JIA who continue anti-inflammatory therapy.9 10 S100A12 (EN-RAGE; calgranulin C), a proinflammatory calcium-binding protein, is highly expressed in inflamed tissue of chronic arthritis. Once released from activated or damaged neutrophils as the paramount cellular source it can operate as an endogenous ligand for toll-like receptor 4 and promotes inflammatory responses.11 12
The primary objective of this study was to test whether in patients with JIA discontinuing treatment, the risk of a flare due to subclinical inflammatory activity can be predicted by combined analysis of the biomarkers S100A12 and hsCRP. We performed a multicentre controlled intervention trial (PREVENT-JIA) with the following stratification of the therapeutic approach: Maintenance therapy for patients with JIA in remission with elevated levels of the biomarkers, stop of therapy if both biomarkers are low. The second major hypothesis of this study was that a risk-stratified decision on withdrawal of therapy is superior to a treatment stop time-point based solely on the clinicians’ perspective (regarding the prevention of flares). We therefore compared patients in the intervention arm to patients in the German registry for biologics in paediatric rheumatology (Biologika in der Kinderrheumatologie (BiKeR)) pharmacovigilance registry who also stopped therapy in remission, but without any stratification.13
Patients and methods
Patient recruitment
The PREVENT-JIA trial recruited patients from May 2013 to October 2018, with subsequent follow-up monitoring until September 2019 in six international paediatric rheumatology centres (University of Muenster, Germany; Children’s Hospital Sankt Augustin, Germany; Cincinnati Children’s Hospital Medical Center, USA; BC Children’s Hospital, University of British Columbia, Vancouver, Canada; Wilhelmina Children’s Hospital, UMC Utrecht, The Netherlands; Riga Stradins University Children’s Hospital Riga, Latvia). The study was reported to the International Standard Randomised Controlled Trial Number registry.
Patient inclusion criteria
Patients from all JIA categories were able to participate, except for those with persistent oligoarthritis or with systemic features within 1 year prior to inclusion. The patients were included after achieving clinical remission on medication (including either conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and/or biological DMARDs (bDMARDs)) and inactive disease for minimum of 6 and maximum of 12 months.14 During the time of clinical remission on medication, medication and dosage remained unchanged (online supplemental tables 1 and 2)(Suppl. Tables S1, S2). A total of 114 out of 119 screened patients fulfilled these inclusion criteria. Prior to reaching the intervention phase, 12 patients were excluded because of protocol deviations, and 2 more were excluded because of a disease flare. Thus 100 patients comprised the PREVENT-JIA group and were eligible for intervention. Details of the patients are summarised in table 1 and online supplemental table 3.
Supplemental material
Demographic data of all PREVENT-JIA participants that were recruited at six centres
PREVENT-JIA study group interventions
The PREVENT-JIA trial is a two-part open-label clinical trial where patients were monitored for 6 months to confirm the presence of clinical remission on medications (part 1) followed by an intervention phase with biomarker-guided withdrawal decisions (part 2). At the intervention time points, a serum sample was sent to the laboratory of the University of Muenster for central analysis of S100A12 and hsCRP. If biomarkers were elevated, patients were continued on treatment until the next 3-month assessment; or if biomarker levels were low, treatment was discontinued. Once treatment was discontinued (or after 12 months for patients with persisting elevated biomarkers and no relapse) they entered the follow-up phase (figure 1). In all cases monitoring was continued for 1 year with regular visits every 3 months after stopping medication. If S100A12/hsCRP stayed above the threshold over the whole study period, therapy was continued for 18 months in total. Afterwards, the decision to continue or stop medication was left to the local physician.
Overview of PREVENT-JIA study interventions. The patients entered the intervention phase after achieving clinical remission on medication, and their serum tested for S100 and C-reactive protein. Further on, at successive 3-monthly intervals for 1 year, each patient was assessed for clinical remission. Both biomarkers needed to be under their respective cut-off for the recommendation to stop treatment, a single one above its cut-off lead to continuing medication. If biomarkers were elevated, patients were continued on treatment until next 3-month assessment; or if biomarker levels were low treatment was discontinued. Once treatment was discontinued (or at 12 months for patients with persisting elevated biomarkers and no relapse) they entered the follow-up phase. JIA, juvenile idiopathic arthritis.
Control group from the BiKeR registry
The control cohort was obtained from the BiKeR (‘Biologika in der Kinderrheumatologie’) registry, a German cohort of patients with JIA treated with csDMARDs or bDMARDs.15 In the control group therapy was withdrawn as per the local physician’s decision and according to the standard of care. In the BiKeR registry, patients in all JIA categories (except persistent oligoarthritis or systemic disease) were screened and a total of 1477 patients with a complete set of data were identified. Among these patients 430 reached clinical remission on medication, remained inactive for at least further 6 months on medication and reached the corresponding first intervention time point of the PREVENT-JIA study. One hundred and eighteen of 430 patients stopped treatment with csDMARDs or bDMARDs between 6 and 18 months after achieving remission; that is, corresponding to the intervention phase of the PREVENT-JIA study (table 2).
Details of the PREVENT-JIA group and the respective BiKeR control patients
Analysis of S100A12 and hsCRP
S100A12 was measured at the Department of Pediatric Rheumatology and Immunology, University Children’s Hospital Muenster, Germany. Until June 2015, quantification was achieved by an ELISA based on polyclonal antibodies as reported previously.9 10 The threshold for low versus high risk of relapse for this assay was at 175 ng/mL.10 After June 2015, a new assay based on monoclonal antibodies with markedly improved sensitivity within low S100A12 serum levels was built in-house.16 The cut-off for discontinuation of treatment changed from 175 ng/mL to 75 ng/mL with the newly validated assay. Samples were remeasured to confirm that the previous cut-off based decisions remained valid. hsCRP was analysed in the central laboratory of the University Hospital by nephelometry. The threshold for low versus high risk of relapses was set throughout the study at 0.3 mg/dL.17
Outcome and endpoints
Time from therapy withdrawal (including both csDMARD and/or bDMARD) to first flare (cumulative flare rate after therapy withdrawal) was defined as primary outcome. Flare was defined as occurrence of any sign of active disease. Patients were censored if they remained inactive for more than 1 year following therapy withdrawal or if they were lost to follow-up. The primary statistical analysis included all patients who entered the intervention phase (at time point I1) and stopped therapy at I1 or within 1 year at one of the subsequent intervention time points.
Time from start of the intervention to first flare (cumulative flare rate after start of the intervention) was defined as key secondary outcome. Patients were censored if they remained inactive for more than 1 year after therapy withdrawal or if they were lost to follow-up. Additionally cumulative medication use over time was investigated as secondary outcome.
Statistics
Baseline demographics and disease characteristics were summarised as mean and SD or median and range, as appropriate, for numerical variables, and frequencies and percentages for nominal and categorical variables. For the primary endpoint analyses, as matching was not possible because of low numbers of available control patients, multivariate regression analyses were performed in order to adjust for possible confounders. The multivariate Cox model with the dependent variable time from therapy withdrawal to first flare included the JIA subtype, type of final therapy (type 1: csDMARD without additional bDMARDs, type 2: bDMARDs with or without additional csDMARD) and duration of therapy (time until inactive disease on stable medication). The Wald test on a significant intervention effect was prespecified as the primary hypothesis test on a (two-sided) 5% significance level to provide confirmatory statistical evidence.
The statistical intention-to-treat analysis of the key secondary outcome included all study patients who entered the intervention phase (n=100). The control group was formed from 430 eligible patients from the BiKeR registry who reached clinical remission on medication and remained inactive for at least further 6 months on medication. Intervention and control patients were matched in a 1:1 ratio in order to adjust for confounders that may bias the comparison of the groups. Control patients were selected so that the JIA subtype and the type of final therapy of a pair of individual patients matched exactly (final therapy type 1: csDMARD without additional bDMARDs, type 2: bDMARDs with or without additional csDMARD), and the duration of therapy matched as well as possible (duration from treatment start until inactive disease on stable medication). The matching algorithm selected pairs of PREVENT-JIA and BiKeR patients so that the sum of absolute differences of therapy durations of two matching partners is minimal among all possible pairings.18
Statistical analyses were performed using SAS (V.9.4 for Windows, SAS Institute) and the R software, package optmatch (https://www.R-project.org).
Results
Of the 100 PREVENT-JIA participants entering the intervention phase, 82 had biomarker levels below the predefined cut-offs and thus stopped medication at the first intervention time point (figure 2, online supplemental fugure 1). Of these 82 patients, 34 individuals (41%) had a flare, mostly during the first 6 months of observation (table 3). Forty-eight patients (59%) remained without flare after stopping medication at I1. At the other intervention time points I2–I4, there was a decision to stop medication in additional nine patients and among these seven patients had a subsequent flare. Eight patients had a disease flare during the next 12 months despite ongoing therapy. The total flare rate in the period from start of the intervention up to the end of the 1 year follow-up phase in the PREVENT-JIA study group thus was 49% (49 of 100 patients). This was significantly lower in comparison to the matched patients from the BiKeR registry showing a total flare rate of 62% (table 3). The number needed to treat (NNT) for avoiding one JIA flare by measuring biomarkers during the intervention phase is NNT=8. After stopping medication patients in the PREVENT-JIA group experienced fewer flares (41 of 91 patients; 45%) compared with patients in the BiKeR registry (15 of 25 patients; 60%) (table 3).
Patient distribution in the PREVENT-JIA intervention group. A total of 100 patients reached the intervention phase and decision-making depending on S100A12/high-sensitivity C-reactive protein levels. In 91 cases medication was stopped; 41 of those experienced a flare in the following 12 months. Out of 18 patients with predicted high risk and a decision to continue therapy after intervention time point I1, a total of eight patients had a flare under medication. JIA, juvenile idiopathic arthritis.
Outcome of the intention-to-treat analysis (n=100 in both groups)
The primary outcome of the study addressed flares that occurred after withdrawal of therapy. Ninety-one PREVENT-JIA patients (figure 2) and 118 patients from the BiKeR registry stopped treatment with csDMARDs or bDMARDs within the 1 year intervention phase. The time from therapy withdrawal to first flare (cumulative flare rate after therapy withdrawal) revealed a significant difference in favour of the PREVENT-JIA study cohort (figure 3; p=0.0455; HR 0.62, 95% CI 0.38 to 0.99, adjusted by JIA subtype, type of final therapy and duration of therapy). Patients in the PREVENT-JIA study revealed a significantly lower cumulative flare rate following therapy withdrawal and therefore a significantly longer time from stopping medication until first flare compared with patients from the BiKeR registry. Differences in the characteristics of the PREVENT-JIA group and the BiKeR cohort (table 2) have to be considered in the interpretation.
Cumulative flare rate after therapy withdrawal. A number of 91 PREVENT-JIA patients and 118 patients from the BiKeR registry stopped treatment with conventional synthetic disease-modifying anti-rheumatic drugs or biological DMARDs within the 1-year intervention phase. Patients in the PREVENT-JIA study revealed a significantly lower cumulative flare rate after therapy withdrawal and therefore a significantly longer time from stopping medication until first flare compared with patients from the BiKeR registry. BiKeR, Biologika in der Kinderrheumatologie; JIA, juvenile idiopathic arthritis.
The key secondary outcome of the study addressed flares that occurred after the start of the intervention. A number of 100 PREVENT-JIA patients reached the first intervention time point I1 and were compared with 100 matched patients from the BiKeR registry. The time from I1 to first flare (cumulative flare rate after the start of the intervention) revealed a significant difference in favour of the PREVENT-JIA study cohort (figure 4; p=0.0371; HR 0.67, 95% CI 0.45 to 0.98, adjusted by JIA subtype, type of final therapy and duration of therapy). Patients in the PREVENT-JIA study revealed a significantly lower cumulative flare rate following the first intervention time point I1 and therefore a significantly longer time from I1 until first flare compared with patients from the BiKeR registry.
Cumulative flare rate after first intervention time point I1. A number of 100 PREVENT-JIA patients reached the first intervention time point I1. Of the 430 eligible patients from the BiKeR registry who reached clinical remission on medication, remained inactive for at least further 6 months on medication and reached the corresponding first intervention time point I1, 100 patients were selected to achieve an exact match in JIA subtype, type of final therapy (final therapy type 1: csDMARD without additional bDMARDs, type 2: bDMARDs with or without additional csDMARD), and the duration of therapy (duration from treatment start until inactive disease on stable medication) between a pair of one PREVENT-JIA and one BiKeR patient as well as possible. Patients in the PREVENT-JIA study revealed a significantly lower cumulative flare rate after the first intervention time point I1 and therefore revealed a significantly longer time from I1 until first flare compared with patients from the BiKeR registry. bDMARDs, biological disease-modifying anti-rheumatic drugs; BiKeR, Biologika in der Kinderrheumatologie; csDMARDs, conventional synthetic DMARDs; JIA, juvenile idiopathic arthritis.
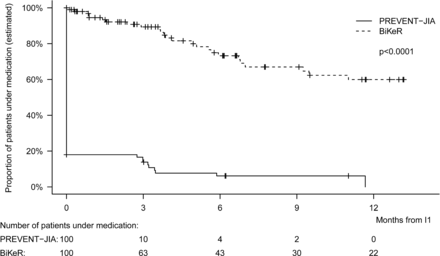
Another secondary outcome was the cumulative medication use over time. It is an important question whether the stratified approach based on biomarkers would lead to prolonged maintenance therapy and thus higher cumulative medication doses, or may facilitate more rapid decisions to stop treatment and thus lower drug exposure. Our analyses revealed that the duration of therapy (time from I1 to stop of therapy) in the PREVENT‐JIA study cohort was significantly reduced compared with the matched BiKeR control group (p<0.0001). In the PREVENT‐JIA group, the majority of patients stopped therapy at I1 (82 of 100 patients=82%), whereas the duration of therapy was significantly longer in the BiKeR control group. In the majority of BiKeR patients (60%) drug therapy was continued for at least 1 year (figure 5).
Duration of therapy. A number of 100 PREVENT-JIA patients reached the first intervention time point I1 and were compared with 100 matched patients from the BiKeR registry. The time from I1 to stop of therapy was significantly reduced in the PREVENT-JIA group compared with the matched BiKeR control group (p<0.0001). In the PREVENT-JIA group, the majority of patients (82%) already stopped therapy at entry into the intervention phase, whereas in the majority of BiKeR patients (60%) drug treatment was continued for at least one further year. BiKeR, Biologika in der Kinderrheumatologie; JIA, juvenile idiopathic arthritis.
Discussion
This study demonstrates that S100A12/hsCRP may work as potential markers of subclinical inflammation and help to evaluate the risk of flares and guide treatment decision. A risk-adapted strategy reduces both number of flares in remission and the cumulative drug exposure compared with standard-of-care practice. Although innovative drugs lead to higher remission rates in JIA, disease remission can often not be sustained long-term.8 19 Persistent subclinical inflammation might explain the occurrence of relapses after treatment withdrawal. However, subclinical inflammation is by definition undetectable by clinical examination and routine laboratory parameters. Our data support the use of biomarker-guided strategies to steer therapy withdrawal.
We have previously demonstrated in retrospective analyses that S100A12 and hsCRP may help to identify patients at risk for flares, which could support decisions to taper, stop or maintain MTX treatment in different scenarios in JIA.9 10 Further analyses showed that S100A12 levels were only moderately correlated with the time to disease flare after discontinuation of anti-tumour necrosis factor therapy.19 We now confirm that S100A12 and hsCRP may contribute to define an immunological remission, although this limited biomarker set cannot definitely exclude flare risks. Nonetheless all patients continuously presenting with S100A12 and hsCRP levels above the defined cut-off flared in the observation period.
The evidence for biomarkers or ultrasound in predicting disease flare is still unclear and controversial. In addition to use of soluble biomarkers of inflammation as described here, the use of molecular signatures of inflammation have also been investigated with partially promising results.20 21 Studies employing transcriptional profiles of peripheral blood cells indicated that clinical remission may not lead to a restoration of immunological normality.20 21 In adults with rheumatoid arthritis, inflammation-related lymphocytes have been shown to persist even in remission, and their frequency associates with the relative risk for relapses.22 23 Specific lymphocyte phenotypes have also been described in patients with JIA prior to flares.24
Data analyses of disease trajectories in larger inception cohorts and observational studies have corroborated a drug-specific risk for relapses.3 25 Patients withdrawn from bDMARDs frequently experience flares, but this effect was smaller if patients remained on medication for more than 2 years.26 From physician surveys we can conclude that considerations for discontinuing medications frequently include disease duration before and under immunosuppression, JIA-disease related damage, JIA subcategory, results of musculoskeletal ultrasound or MRI, tapering versus immediate medication withdrawal, as well as medication selection (csDMARD, bDMARDs, combinations) and also different laboratory markers.27 Yet, no robust uniform factors could be identified. In the German BiKeR registry (included in our study as a control group) reoccurrence of active disease was reported for 77% of patients after stopping etanercept, with mean time to flare of 1 year. In the BiKeR patients, it was not possible to identify any factor correlating to flare risk.28 Overall, it seems easier to predict complications in active disease phases than to confirm long-term stability of disease remission.29 30
Past studies on treatment withdrawal were criticised for their observational design and low quality.31 We thus designed a prospective trial including a defined study population with controlled disease duration. We found a significant reduction in both flare rates and cumulative drug usage with the stratified approach, as compared with real-life data from the BiKeR registry. It is conceivable that predefined treatment-withdrawal strategies will result in a higher likelihood of successful flare-free outcome and less medication use. However, a selection bias towards patients with optimal stability of remission at inclusion cannot be excluded. This is also shown by a relatively small overall impact of the biomarker-driven stratification, as most patients had no indication of subclinical inflammation. Moreover, enrolment in a study with frequent follow-up visits in course of clinical remission may already benefit overall treatment and outcome.
An important limitation of our study is the relatively small patient number. Most patients included in the PREVENT-JIA trial had rheumatoid factor negative polyarthritis. We therefore draw conclusions for predominantly this subgroup. Therapy with bDMARDs might have a distinct effect on biomarker expression, which may impact the ability of S100A12 and hsCRP to detect subclinical inflammation.19 There were fewer patients with bDMARDs in the PREVENT-JIA study cohort compared with the BiKeR control group and literature suggests that there is a higher flare risk following withdrawal of bDMARDs versus other agents.19 Our study demonstrated a trend towards a higher flare rate after stopping treatment with bDMARDs versus stopping csDMARDs, but was insufficiently powered to be conclusive. Future studies specifically on patients receiving bDMARDs compared with csDMARD might therefore be reasonable. A second limitation is given by the differences between the PREVENT group and the control cohort according to population characteristics, their disease severity or treatment approaches that may contribute to a better outcome of the PREVENT group. The BiKeR registry is an active project collecting real-life data, constantly recruiting patients who are followed-up systematically. Nonetheless, it has no specific treatment/intervention plan and has therefore unavoidable differences to the data collection in the PREVENT study.
Taken together, decision-making for therapy withdrawal in clinical remission of JIA remains challenging. Most patients entering this trial had no biomarker-proven subclinical inflammation and could stop therapy rapidly. The decision-making process, using markers of subclinical inflammation with the potential to predict the further course, led to a higher proportion of patients in whom drug treatment was stopped, and it significantly reduced the overall flare rate. Despite limitations, primarily due to a relatively small number of enrolled individuals, our data indicate that managing patients with inactive disease in a stratified approach offers a benefit for clinical practice.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethics Committee Westphalia-Lippe under reference number 2011-079-f-S. Participants and/or legal representatives gave informed consent to participate in the study before taking part.
Acknowledgments
The authors are especially grateful to all patients and their parents for their participation in PREVENT-JIA study. The authors also thank all physicians engaged in the study. We thank Melanie Saers, Sabrina Fuehner and Susanne Schleifenbaum for technical assistance. We are also grateful to Antje Hellige for excellent study coordination and monitoring.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Handling editor Josef S Smolen
Correction notice This article has been corrected since it was first published. The open access licence has been updated to CC BY. 25th May 2023.
Contributors JG, MT and DF analysed and interpreted the data and wrote the manuscript. MM-G and DH performed the data acquisition. GH and AK provided data form the BiKeR data base. CK designed the monoclonal S100A12 assay and supervised laboratory measurement. JG designed and performed the statistical analyses. GH, JFS, VS, DAC and HIB conducted the trial and contributed in writing the manuscript. All authors read and approved the final manuscript. DF accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding The PREVENT-JIA study received intramural funding by a Clinical Research Award from the Interdisciplinary Center of Clinical Research at the University of Muenster (IZKF project CRA-004).
Competing interests JG has received honoraria from TESARO, QUIRIS Healthcare, Ecker+Ecker, Dr August Wolff, Roche, University Clinics Schleswig‐Holstein and RWTH Aachen University, Germany; GH has received research grants from Pfizer, Merck Sharp Dome, Novartis and Roche and honoraria from Bayer, Chugai, Janssen, Pfizer, Sanofi, Sobi Swedish Orphan and Novartis; CK has received honararia from Novartis and SOBI and research support from Novartis; JFS has received consultancy fees from Amgen; HIB has received speaking fees from Novartis, Pfizer and GlaxoSmithKline and consultancy fees/honoraria from AbbVie, Astra Zeneca-Medimmune, Biogen, Boehringer, Bristol Myers Squibb, Celgene, Eli Lilly, EMD Serono, Idorsia, Cerocor, Janssen, GlaxoSmithKline, F Hoffmann-La Roche, Merck, Novartis, R-Pharm and Sanofi, while The Cincinnati Children’s Hospital, where HIB works as a full-time public employee, has received contributions from Bristol Myers Squibb, F Hoffmann-La Roche, Janssen, Novartis and Pfizer; DF has received research grants from Pfizer, Sobi Swedish Orphan and Novartis, and he has received speaking fees from Sobi Swedish Orphan, Novartis and Werfen as well as consultancy fees/honoraria from Boehringer; The other authors declare that they have no competing interests.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.