Article Text
Abstract
Secukinumab, ixekizumab and brodalumab are monoclonal antibody therapies that inhibit interleukin (IL)-17 activity and are widely used for the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. The promising efficacy results in dermatology and rheumatology prompted the evaluation of these drugs in Crohn’s disease and ulcerative colitis, but the onset of paradoxical events (disease exacerbation after treatment with a theoretically curative drug) prevented their approval in patients with inflammatory bowel diseases (IBDs). To date, the pathophysiological mechanisms underlying these paradoxical effects are not well defined, and there are no clear guidelines for the management of patients with disease flare or new IBD onset after anti-IL-17 drug therapy. In this review, we summarise the literature on putative mechanisms, the clinical digestive effects after therapy with IL-17 inhibitors and provide guidance for the management of these paradoxical effects in clinical practice.
- arthritis, psoriatic
- spondylitis, ankylosing
- biological therapy
Statistics from Altmetric.com
Introduction
Several biologics, including tumour necrosis factor (TNF) inhibitors and ustekinumab, are approved for gastroenterological,1–3 rheumatological and dermatological4 5 inflammatory conditions. However, about 30%–40% of patients treated with these drugs do not respond to treatment (primary non-response) or lose response over time (secondary non-response), suggesting the need for new drugs.6 Interleukin-17 (IL-17) inhibitors have emerged over the past decades as effective treatments for ankylosing spondylitis (AS) and psoriatic arthritis (PsA)7–9 by improving disease activity and patients’ quality of life.10–24 Three monoclonal antibody therapies targeting IL-17A (secukinumab and ixekizumab) and IL-17 receptor (brodalumab) are currently available, but their use has been associated with the onset of paradoxical effects,25 defined as the appearance or exacerbation of the inflammatory disease after therapy with a drug generally used to treat specific pathological conditions.26 To date, paradoxical events have been reported after treatment with anti-IL17 drugs,26 but limited data are available concerning their cumulative incidence and risk factors.27 The mechanisms underlying these events are not well understood but might involve type I interferon derived from plasmacytoid dendritic cells, resulting in a hyperactive innate inflammatory process.28 Interestingly, type I interferon overexpression is responsible for the skin phenotype of paradoxical psoriasis, which is the most frequent event in patients treated with anti-TNF agents.28 Notably, as most paradoxical events usually extend to the entire class of drugs, drug withdrawal is often necessary.29 The elevated levels of IL-17A within the lamina propria of patients with Crohn’s disease (CD) suggested this cytokine could be a therapeutic target in patients with inflammatory bowel disease (IBD).30 31 Similarly, independent large Genome-Wide Association Cohort Studies (GWAS) implying the IL-23–IL-17 axis in disease pathogenesis32 33 prompted researchers to investigate the safety and efficacy of secukinumab in CD.34 Experimental colitis murine models revealed conflicting data on the role of the IL-23–IL17 pathway, with neutralisation leading to worsening or healing of intestinal inflammation.35 36 This dual effect has been recently explained as anti-IL23 antibodies reducing T helper (Th) 17 autoimmunity, thereby improving colitis, whereas IL-17 neutralisation, which affects tissue homeostasis repair, impairs intestinal wall integrity and exacerbates disease.37 Clinical trials suggested that anti-IL-17 might worsen bowel inflammation in patients with IBD, halting the development of this drug class.34 38 Physicians should be cautious when prescribing IL-17 blockers in patients with IBD or personal history suggestive of IBD.39 40 Nonetheless, no specific guideline is available to simplify the therapeutic decisions of dermatologists and rheumatologists. The aim of this review was therefore to assess all published cases of digestive paradoxical effects after therapy with IL-17 inhibitors to better define these events and to provide guidance for clinical practice.
Search strategy and selection criteria
We searched on PubMed, Embase and Web of Science up to March 2020 all studies on digestive paradoxical effects. The following search terms were used : “paradoxical effects”, “ interleukin-17 blockers”, “anti-IL-17”, “ secukinumab”, “ixekizumab”, “brodalumab”, “ixekizumab”, “inflammatory bowel disease”, “Crohn’s disease”, “ulcerative colitis”, “spondyloarthritis”, “psoriasis” and “ankylosing spondylitis”. No language restriction was applied. We focused on full-text articles, although relevant abstracts were considered. In addition, further studies were identified through the accurate evaluation of the reference lists of the assessed manuscripts. Finally, the studies were included in our review on the basis of originality and relevance.
Molecular aspects
What is IL-17?
In 2005, a third subset of Th lymphocytes was identified, in addition to the previously recognised Th1 and Th2 subsets. This third subset promotes the expression of IL-17A, IL-17F, IL-21 and IL-22 and was termed Th17 cells (figure 1).41 IL-17A and IL-17F are proinflammatory cytokines produced by activated Th17 cells that induce the NF-κB and mitogen-activated protein kinases signalling pathways.41 IL-17A stimulates expression of IL-6 and cyclo-oxygenase 2, and enhances the production of nitric oxide in many cells, expressing the heterodimer receptor formed by IL-17 receptor A and IL-17 receptor C.42 Th17 lymphocytes and IL-17 are involved in the immune response against extracellular pathogens through the regulation of intestinal epithelial permeability and act more specifically in the mucosal interface.43 44 In basal conditions, most Th17 cells are found in the lamina propria of the gastrointestinal wall.45 These cells protect against bacterial and fungal infections, but their dysregulation can promote the development of autoimmune diseases such as multiple sclerosis, IBD, psoriasis and arthritis.46
Molecular aspects of the IL-17 pathway (A) regulation of IL-17 production several cells are involved in IL-17 production and selective inhibition of IL-17 or IL-23 can have different effects on the immune response (B). Role of IL-17 in intestine and joints inhibition of IL-17 causes a reduction in joint inflammation, but at the same time negatively affects the activity of the intestinal barrier by worsening gut inflammation (C). IL-17 signaling pathway the different IL-17 subpopulations elicit different intracellular responses through binding to specific receptors. AS, ankylosing spondylitis; IL-17, interleukin-17; IL-17RA/C, IL-17 receptor A/C; iNKT, invariant natural killer T cell; LCN2, lipocalin-2; OCL, osteochondral lesions; PsA, psoriatic arthritis.
Role of IL-17 in immune-mediated inflammatory diseases
Inflammatory bowel disease
Ulcerative colitis (UC) and CD have an annual incidence ranging from 0 to 19.2 and 0–20.2 per 100 000 persons in North America to 0.6–24.3 and 0.3–12.7 per 100 000 persons in Europe.47 IBD pathogenesis is related to an excessive and uncontrolled immune response against normal microbiota, through the activation of CD4+ Th cells (figure 2).48 Classically, CD is primarily mediated by Th1 cells and UC by Th2 cells, but it is now well established that Th17 cells and their related cytokines are crucial mediators in both conditions.45 Increased levels of IL-17, Th17 cells and Th17-related cytokines were found in the gastrointestinal mucosa of patients with IBD.45 49 In addition, IL-17A mRNA concentration is higher in bowel mucosa of patients with IBD and an association between IBD and polymorphisms in Th17-related genes, such as STAT3 or IL-23R, has been demonstrated in GWAS.32 33 The massive infiltration of Th17 cells in the inflamed intestine of patients with IBD leads to the production of IL-17A and other cytokines, triggering and amplifying the inflammatory process.45 In line with this finding, several studies showed an increase of IL-17A and IL-17F mRNA in patients with IBD.30 31
The joint-gut axis the pathophysiological model of the relationships between joint inflammation and IL-17 can be extrapolated from psoriasis. IL-17, interleukin-17.
Inflammatory joint disease
IL-17A, IL-17F and IL-22 might also have a fundamental role in arthritis pathogenesis, as their neutralisation is associated with reduced disease severity in both experimental50 and human studies.51 The role of IL-17 in AS was initially supported by the finding of several polymorphisms in genes involved in generation and maintenance of Th17 lymphocytes.33 Interestingly, these genes are also associated with IBD and psoriasis.46 Increased levels of circulating Th17 cells were found in patients with rheumatoid arthritis (RA), although treatment with IL-17 inhibitors resulted in only a modest clinical response.52–54 On the other hand, Th17 cells and their effectors (neutrophils) were identified in tissue samples from facet joints of individuals with AS and the inhibition of IL-17 was recognised as a valid therapeutic option for the management of this disease, suggesting the existence of different underlying pathophysiological mechanisms.55 56Studies published in the past few years have revealed the involvement of an IL-17-producing subset of innate immune cells, called ILC3 cells, in both AS and IBD.57 58 Although ILC3 cells are mainly located in the gut mucosa of healthy individuals, where they have a protective role against pathogens and in the maintenance of intestinal homeostasis, their number is increased in patients with AS with ileal inflammation.58 ILC3 cells could also have a role in IBD aetiology but there is no clear evidence in this field currently.58
Relationship between IL-17 and intestinal inflammation in experimental models
As previously mentioned, IL-17 is essential in immune response against extracellular pathogens as the recruitment of neutrophils in case of bacterial, yeast, or fungal infections is regulated by IL-17 activity.43 Importantly, the development of anti-IL-17 antibodies results in an increased susceptibility to infections in patients with autoimmune diseases.59 In experimental colitis animal models, neutralisation of IL-17A or IL-17F by genetic ablation or by antibody treatment led to severe weakening of the intestinal epithelial barrier and worsening of intestinal inflammation.35 36 Conversely, another experimental mouse model showed that colitis was associated with an increase in IL-17A levels, while IL-17F values were inversely correlated with gut inflammation.60 Interestingly, colitis was not improved by the single inhibition of IL-17A or IL-17F, whereas the combined blockade of both cytokines prevented the development of CD4/CD25 transfer induced colitis, suggesting that IL-17A and IL-17F may share some proinflammatory functions.60 Furthermore, the inhibition of IL-22 activity in an UC mouse model led to reduced production of mucus by the intestinal goblet cells, aggravating inflammation and suggesting a tissue-protective role for this cytokine.61 Th17 cells are prominent in intestine mucosal surfaces, where they contribute, in cooperation with other T cells, to maintain intestinal homeostasis and protect against microorganisms.62 The concentration of intestinal Th17 cells is reduced in mice treated with antibiotics and in germ-free mice,63 64 whereas it is increased 2 weeks after faecal transplantation from control mice to germ-free mice.65 Accordingly, the inoculation of Gram-positive spore-forming bacteria in the gut lamina propria of germ-free mice promoted the induction of Th17 cells.65 For this reason, IL-17 suppression might interfere with its gut protective function, causing the onset of infections and explaining the clinical failure of anti-IL-17 drugs in CD treatment.34
Clinical aspects
Clinical trials
Anti-IL-17 agents have been tested in patients with IBD and those with rheumatological and dermatological diseases (table 1). In a randomised, double-blind, placebo-controlled phase II trial, 130 patients with CD were randomised 1:1:1:1 to receive brodalumab (at a dosage of 210, 350 or 700 mg) or placebo at baseline and at week 4. Remission rates were higher in patients treated with brodalumab 210, 350 or 700 mg than in the placebo group (3%, 15%, and 9% vs 3%, respectively, p<0.05).38 Similarly, a greater Crohn’s Disease Activity Index (CDAI) response at week 6 occurred in brodalumab group than in the placebo group (16%, 27% and 15% vs 13%, respectively, p<0.05).38 However, there was no statistically significant difference in mean CDAI change from baseline at week 6 among the groups and CD worsening was detected in a higher rate of patients in anti-IL-17 group compared with the placebo group (25.0% vs 6.3%), leading to the early termination of the study.38 Another randomised, double-blind, placebo-controlled phase II trial investigated the efficacy and safety of secukinumab in 59 patients with CD.34 Eighteen patients (31%) terminated the study prematurely and a preplanned preliminary analysis showed that mean reduction in the CDAI score at week 6 was greater in patients treated with placebo compared with secukinumab (–63.1 points vs –29.2 points, p=0.043).34 Adverse events and serious adverse events occurred more in the secukinumab than in the placebo groups (74% and 28% vs 50% and 10%, respectively), causing early study interruption.34
Characteristics and results of clinical studies on anti-IL-17 drugs
Clinical trials of anti-IL-17 therapies in rheumatology and dermatology also reported some cases of newly diagnosed IBD or IBD exacerbations. A systematic review and meta-analysis of 38 randomised clinical trials66 evaluated the risk of new onset IBD with the use of anti‐IL‐17 agents (brodalumab, secukinumab and ixekizumab) in patients with psoriasis, PsA, RA and AS. In total, 12 new IBD events (5 CD and 7 UC) were found during a 60-week follow-up in the 16 690 subjects treated with anti-IL-17 drugs, corresponding to an incidence rate of 2.4 per 1000 patient‐years. No new IBD case was registered with brodalumab treatment, whereas 4 and 8 cases were reported with ixekizumab and secukinumab, respectively,.66 In another work, one CD case was reported with brodalumab (n=1576) and 4 CD and 7 UC with ixekizumab (n=3736) for moderate-to-severe psoriasis. Ixekizumab every 2 weeks led to a moderate exacerbation of UC in one patient and new CD onset in one patient.67 A pooled analysis of 21 clinical trials including 7355 patients investigated the IBD incidence in patients treated with secukinumab.68 Among 5181 individuals with psoriasis, 20 were diagnosed with IBD (14 UC, 5 CD and 1 indeterminate colitis (IC)), inclusive of 14 new cases.68 IBD was detected in 8 out of 1380 patients with PsA (3 UC, 3 CD and 2 IC) of which 7 cases were newly diagnosed.68 Four UC, 8 CD and 1 IC were found in 794 AS patients with nine new cases, suggesting a low overall rate of IBD events in these patients.68 MEASURE 1, 2 and 3 trials and their extension studies were phase III trials assessing the efficacy and safety of secukinumab in AS patients .10–14 16 69 This anti-IL-17A monoclonal antibody proved to be effective for the treatment of AS, but an exposure adjusted incidence rate of IBD ranging between 0.2 and 0.8 events per 100 patients-years was found. The 4 year results from the MEASURE 1 study revealed the onset of 7 CD and 2 UC cases in AS patients, underlining that some individuals had no personal or familial history of IBD and no digestive symptom before secukinumab therapy.13 A phase 3, randomised, double blind study by van der Heijde et al 70 reported ixekizumab safety data for the treatment of AS, showing only one new CD case among the 164 treated patients over a 1-year follow-up. A pooled analysis from seven clinical trials provided short-term and long-term safety data for ixekizumab in psoriasis.18 The overall rate of CD and UC patients was low, accounting for one patient with newly diagnosed CD, one patient with UC exacerbation during the induction phase, and four patients with UC flares during the maintenance therapy. Finally, a recent systematic review and meta-analysis compared the risk of IBD development among patients with psoriasis, PsA and AS exposed and non-exposed to anti-IL-17 drugs (secukinumab, ixekizumab or brodalumab).71 A total of 66 studies were analysed including 14 390 patients treated with IL-17 inhibitors during the induction phase alone or induction and/or maintenance phases. It is noteworthy that no difference in the pooled risk of new-onset IBD was found between experimental and placebo groups during induction and maintenance studies (risk difference (RD)=0.0001 for the best-case scenario, 95% CI −0.0011 to 0.0013 and RD=0.0008 for the worst-case scenario, 95% CI −0.0005 to 0.0022).
Real-world evidence
A total of 19 newly diagnosed IBD cases have been described in case reports after treatment with secukinumab in real-world clinical practice settings36 72–82 and 2 after ixekizumab treatment.83 84 These 21 IBD cases included 10 UC, 6 CD and 5 indeterminate IBD cases. All diagnoses were confirmed by imaging (CT scan or MR enterography (MRE)) and anatomopathologic findings. Characteristics of these case reports are detailed in table 2. Eleven patients were treated for psoriasis, six for AS and four for PsA. None of them had personal history of IBD, although an IBD family history was found in three patients. One patient experienced diarrhoea and weight loss before secukinumab and two had history of abdominal pain (before secukinumab treatment or during tocilizumab respectively). Time before digestive symptoms ranged from 1 week to 56 months, with a mean time of 12.8 months. Alternative treatments to improve gastrointestinal symptoms and to treat the underlying pathology were mainly based on corticosteroids and anti-TNFs (58% of the cases): 10 cases were treated with anti-TNFs (5 infliximab, 3 adalimumab, 1 certolizumab, 1 golimumab), 9 with corticosteroid therapy, 2 with ustekinumab, 2 with mesalazine and 1 with tofacitinib or azathioprine respectively. Secukinumab was stopped in all cases, resulting in substantial improvement of symptoms in all patients. Figure 3 details the case of a 46-year-old women with AS and no personal or familial history of bowel disorders under secukinumab therapy, who was recently admitted to our gastroenterology department at Nancy university hospital for acute severe colitis.
Case report: the patient was followed by rheumatologists since 2015 and initially she was successfully treated with golimumab. in October 2019, after recurrent pulmonary infections, secukinumab was introduced, and in November 2019 (after the third secukinumab injection), the patient presented acute diarrhoea and rectal bleeding for 15 days. The drug was immediately stopped and a colonoscopy was performed, showing diffuse ulcerations from the rectum to the transverse colon and requiring termination of the procedure owing to the high risk of perforation. The infectious tests were negative and corticosteroid therapy (100 mg per day) was initiated, followed by rapid improvement of symptoms. A subtotal colectomy with terminal ileostomy was performed because of the life-threatening situation and the pathology report confirmed the UC aspect (without dysplasia or malignancy). UC, ulcerative colitis.
Characteristics of case reports reporting digestive symptoms in patients treated with secukinumab or ixekizumab
Practical recommendations
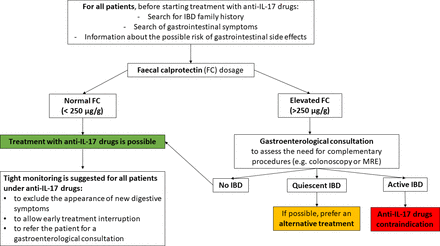
Although the IBD onset can occur in patients who have never experienced gastrointestinal symptoms and have no family members with IBD, a careful search for IBD family history and gastrointestinal symptoms should be undertaken in all patients before starting treatment with IL-17 inhibitors (figure 4).39 40 85 In addition, all patients should be informed of the possible risk of gastrointestinal adverse effects, and as some patients might have subclinical bowel inflammation, faecal calprotectin (FC) levels should be measured to detect gut inflammation, enabling patients to be stratified and guiding physicians' decisions.86 Normal FC values (<250 µg/g87) should authorise treatment with anti-IL-17 drugs, although tight monitoring remains essential to exclude the appearance of new digestive symptoms, to enable early treatment interruption, and to refer the patient for gastroenterological consultation. If FC is elevated (>250 µg/g),87 a gastroenterological evaluation should be indicated to assess the need for complementary procedures (eg, colonoscopy or MRE) and to newly diagnose IBD. In case of confirmed active IBD, anti-IL-17 drugs should be contraindicated, whereas in patients with quiescent IBD, alternative treatments should be preferred. In patients with a suspected diagnosis of IBD, IL-17 inhibitors could be a therapeutic option. However, another treatment (anti-TNF agents, ustekinumab, apremilast) should be preferred. If IL-17 inhibitors are used, tight monitoring is required, and treatment should be stopped in case of diarrhoea or other digestive symptoms as anti-IL-17 agents are associated with an increased risk of serious infections in IBD patients.34
Practical recommendations before anti-IL-17 drug initiation recommendations are based on the experience of the authors. FC, faecal calprotectin; IBD, inflammatory bowel disease; IL-17, interleukin-17; MRE, MR enterography.
Conclusion
Our work shows that treatment with brodalumab, ixekizumab and secukinumab is associated with a low incidence of CD, UC and IC in patients with rheumatological and dermatological autoimmune diseases in both clinical trials and clinical practice (2.4 per 1000 patient‐years). However, the onset of these digestive adverse events cannot be neglected and requires continuous and careful monitoring. The management of these patients should be based on a multidisciplinary approach, involving gastroenterologists, dermatologists and rheumatologists to identify possible side effects related to this class of drugs and to prevent any complications. Finally, longer pharmacovigilance studies and further aetiopathological investigations are necessary to obtain long-term safety data of IL-17 inhibitors and to better understand the mechanisms underlying these events.
Acknowledgments
This work was partly supported by the French PIA project "Lorraine Université d'Excellence", reference ANR-15-IDEX-04-LUE.
References
Footnotes
Handling editor Josef S Smolen
Contributors MF, DM, PN, NP, FD, DA, J-YJ, and DL selected the studies and wrote the article. LP-B conceived and critically revised the manuscript. The manuscript was approved by all authors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests LP-B has served as a speaker, consultant and advisory board member for Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boehringer-Ingelheim, Lilly, HAC- Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, Theravance.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.