Article Text
Abstract
Objective To develop new classification criteria for systemic lupus erythematosus (SLE) jointly supported by the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR).
Methods This international initiative had four phases. (1) Evaluation of antinuclear antibody (ANA) as an entry criterion through systematic review and meta-regression of the literature and criteria generation through an international Delphi exercise, an early patient cohort and a patient survey. (2) Criteria reduction by Delphi and nominal group technique exercises. (3) Criteria definition and weighting based on criterion performance and on results of a multi-criteria decision analysis. (4) Refinement of weights and threshold scores in a new derivation cohort of 1001 subjects and validation compared with previous criteria in a new validation cohort of 1270 subjects.
Results The 2019 EULAR/ACR classification criteria for SLE include positive ANA at least once as obligatory entry criterion; followed by additive weighted criteria grouped in seven clinical (constitutional, haematological, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, renal) and three immunological (antiphospholipid antibodies, complement proteins, SLE-specific antibodies) domains, and weighted from 2 to 10. Patients accumulating ≥10 points are classified. In the validation cohort, the new criteria had a sensitivity of 96.1% and specificity of 93.4%, compared with 82.8% sensitivity and 93.4% specificity of the ACR 1997 and 96.7% sensitivity and 83.7% specificity of the Systemic Lupus International Collaborating Clinics 2012 criteria.
Conclusion These new classification criteria were developed using rigorous methodology with multidisciplinary and international input, and have excellent sensitivity and specificity. Use of ANA entry criterion, hierarchically clustered and weighted criteria reflect current thinking about SLE and provide an improved foundation for SLE research.
- systemic lupus erythematosus
- lupus
- classification criteria
- consensus methods
- multi-criteria decision analysis
- validation
Statistics from Altmetric.com
- systemic lupus erythematosus
- lupus
- classification criteria
- consensus methods
- multi-criteria decision analysis
- validation
This criteria set has been approved by the European League Against Rheumatism (EULAR) Executive Committee and the American College of Rheumatology (ACR) Board of Directors. This signifies that the criteria set has been quantitatively validated using patient data, and it has undergone validation based on an independent data set. All EULAR/ACR-approved criteria sets are expected to undergo intermittent updates. The ACR is an independent, professional, medical and scientific society that does not guarantee, warrant, or endorse any commercial product or service.
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with variable clinical features.1 2 SLE manifestations are associated with multiple autoantibodies, ensuing immune complex formation and deposition, and other immune processes.2 3 This complex clinical presentation and pathogenesis makes SLE a difficult disease to grasp and define. Classification criteria are essential for the identification of relatively homogeneous groups of patients for inclusion in research studies and trials.4 5 The 1982 revised American College of Rheumatology (ACR) SLE classification criteria6 and their 1997 revision7 have been used worldwide. Since then, our understanding of the disease has advanced. Additional specific skin manifestations were described, some clinical symptoms were better understood, and immunological tests, such as diminished levels of serum complement components C3 and C4 or testing for anti-β2 glycoprotein I antibodies, entered routine clinical practice. Better understanding of organ system involvement, such as mucocutaneous abnormalities, led to questions about whether some of the independently counted criteria were in fact manifestations of the same phenomenon.8
The 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria addressed many of these issues.9 Mucocutaneous and neuropsychiatric manifestations were added, as were hypocomplementemia and new antiphospholipid antibody tests; and criteria definitions were refined. The SLICC criteria emphasised that SLE is primarily an autoantibody disease, requiring at least one immunological criterion to be present, and categorised histology-proven nephritis compatible with SLE as sufficient for classification, if antinuclear antibodies (ANAs) or antibodies to double-stranded DNA (dsDNA) were present. While achieving their goal of increasing sensitivity, the SLICC criteria have lower specificity than the 1997 ACR criteria.9 10
Existing SLE classification criteria perform better in patients with longstanding disease than in new-onset SLE,11 and there is an increasing recognition and demand that subjects with early SLE should be included in clinical studies and trials. We therefore attempted to enrich our sample populations for early SLE in several phases of the project.
In parallel with improved understanding of SLE, the field of classification criteria development has also seen advances.4 12–14 In order to minimise investigator bias, it is now recommended that the cohorts in which the criteria are tested are from independent centres.4 Other methodological recommendations include a balanced use of both expert-based and data-driven methods, and inclusion of the patient perspective.13 14 The approach chosen for these 2019 European League Against Rheumatism (EULAR)/ACR SLE classification criteria was specifically designed to maintain this balance and to uphold rigorous methodology.
Methods
Methodological overview
Using a methodological approach based on measurement science the criteria were developed in four phases10: (1) criteria generation, (2) criteria reduction, (3) criteria definition and weighting and (4) refinement and validation (figure 1). The whole initiative was overseen by a 12-member steering committee (MA, KHC, DD, MM, RR-G, JSS, DW, DB, DK, DJ, TD and SRJ) nominated by EULAR and the ACR in equal numbers, based on SLE and/or methodological experience and previous involvement in international projects.
Development and validation of SLE classification criteria. ANA, antinuclear antibody; SLE, systemic lupus erythematosus.
The current project, jointly supported by the EULAR and the ACR, was originally based on two key concepts. One, we hypothesised that the presence of ANA would be better employed as an entry criterion than as a classification criterion.10 Such an approach was thought to reflect underlying SLE pathogenesis, and take into account ANA test characteristics of high sensitivity and limited specificity. Two, we expected individual criteria would not be of equal utility (weight) for the classification of SLE,15 for example, mucosal ulcers versus biopsy-proven lupus nephritis. Accordingly, the validity of using positive ANA as an entry criterion was explicitly addressed in phase I of the current activity.16 Likewise, methodological strategies to develop weighted criteria were used.
Phase I. Criteria generation
The purpose of phase I was to test ANA as a potential entry criterion and identify candidate criteria that should be considered for SLE classification using both data-based and expert-based methods, including the patient perspective. Phase I a comprised a systematic literature review of Medline, Embase and the Cochrane databases with meta-regression to evaluate the operating characteristics of ANA testing for consideration as an entry criterion.16 Phase I b consisted of a Delphi exercise of international SLE experts from the Americas, Europe and Asia.17 These experts included rheumatologists, dermatologists, nephrologists, paediatricians and non-clinical SLE researchers, providing a broad perspective. The Delphi participants were asked to nominate a broad set of items potentially useful in the classification of SLE.17 In round 2 and 3, participants rated the items from 1 (not at all appropriate) to 9 (completely appropriate) for classification of SLE. Criteria were retained if they reached a median rating of ≥6.5; that is, at least 50% of the ratings in the high range (7, 8 or 9). Participants were also asked about the importance of ANA and histopathology for classification of SLE. Phase I c established an international cohort of patients with early SLE or conditions mimicking SLE to identify criteria that may discriminate subjects with early (less than 12 months) disease.18 Phase I d comprised a cross-sectional survey of SLE patients, administered via the quarterly journal of the German SLE patient organisation, which asked about symptoms within 1 year before and after the patient’s diagnosis of SLE.19 While at a risk of recall bias and not necessarily representative of other regions worldwide, this survey was done to explicitly take a patient standpoint into account.
For phase II and III, additional renowned European and North American SLE experts were nominated by the steering committee and invited to participate.
Phase II. Criteria reduction
Phase II a. The objective of this phase was to select a set of criteria from phase I that maximised the likelihood of accurate classification of SLE, particularly of early disease. An independent panel of seven of the international SLE experts (RC, NC-C, DDG, BHH, FH, EM and JS-G) ranked the candidate criteria from phase I. A consensus meeting of 19 international SLE experts (n=7 nominal group technique (NGT) experts+steering committee+DK (moderator)) using NGT was conducted to reduce the list of criteria.20 Data for each candidate criterion were reviewed and discussed until consensus was achieved. The NGT experts voted on items to be retained. Phase II b. NGT participants pointed out that some criteria could be correlated. With the idea of potentially clustering criteria into domains, associations between candidate criteria were evaluated separately in two cohorts, the phase Ic early SLE and the Euro-lupus cohorts.21
Phase III. Criteria definition and weighting
Phase III a. The operating characteristics of the retained candidate criteria were evaluated by literature review. Candidate criteria were hierarchically organised into clinical and immunological domains, and definitions for the candidate criteria were iteratively refined. SLE patient advocates participated in the review of data and the steering committee discussions.22
Phase III b. 164 case vignettes reflecting broad SLE clinical presentation were sampled from SLE centres across several countries. A panel of six of the international experts not involved in earlier phases of the project (BD, SJ, WJMcC, GR-I, MS and MBU) and 11 members of the steering committee assessed and ranked a representative sample of the cases. Subsequently, at a face-to-face meeting, this panel of 17 international SLE experts iteratively compared pairs of criteria, using multicriteria decision analysis facilitated by 1000minds software.23 The panel unanimously agreed to further reduce the list of criteria. Based on the results, provisional criteria weights were assigned and a provisional threshold score for classification was determined as the lowest score at which the expert panel had achieved consensus on classifying a case vignette as SLE.24
Phase IV. Refinement and validation
International SLE experts not involved in phase II or phase III panels were asked to contribute cases diagnosed as SLE and controls with conditions mimicking SLE sampled from patients evaluated at their centres. Each centre was asked to contribute up to 100 cases and an equal number of controls, preferentially sampling those with early disease, and regardless of their specific clinical or immunological manifestations. Pseudonymised data on the criteria were collected using a standardised data collection form. Ethics committee approval and informed consent were obtained as per local requirements. The status (‘SLE’ or not) of each case underwent independent adjudication by three of four SLE experts (GB, BFH, NL and CT) from different centres. Queries were sent back to the submitting investigator for clarification. Of this cohort, 501 SLE and 500 control subjects were randomly selected to comprise the derivation cohort, while the remaining 696 SLE and 574 control subjects formed the validation cohort.
Refinement. The performance of the draft criteria set was iteratively tested in the derivation cohort. A data-driven threshold for classification was determined by receiver operating characteristics (ROC) analysis and compared with the provisional expert-based consensus threshold. The data of SLE subjects below the threshold (misclassified) were reviewed for groups of patients with unequivocal SLE who still missed classification, and criteria weights adjusted slightly, while preserving the weighting hierarchy (details below in Results, Phase IV section). Sensitivity and specificity was tested against the ACR 1997 and the SLICC 2012 criteria. In addition, ANA as an entry criterion was tested against not having an entry criterion. Finally, the criteria weights were simplified to whole numbers. Refinements to the criteria set were presented to the steering committee and phase III expert panel, and unanimously endorsed.
Validation. The sensitivity and specificity of the final criteria were tested in the validation cohort and compared with previous SLE criteria sets.
Statistical analysis. Descriptive statistics were used to summarise the data. CIs were calculated using the bias-corrected and accelerated bootstrap method (BCa method) with B=2000 bootstrap samples. The BCa method resamples the input data B times (with replacement) and calculates the required statistics (sensitivity, specificity, area under the curve (AUC)). Based on the B bootstraps samples, the bias-correction is applied and the associated 95% CIs for the statistics are estimated. The BCa method has proven to yield very accurate coverage of estimated CIs.25 The number B of bootstrap resamples is recommended to be at least B=1000. We have chosen B=2000 and additionally checked if B=5000 bootstraps changed the estimated confidence bounds, which was not the case. Statistical analyses were performed using R, V.3.4.0 (The R Foundation of Statistical Computing).
Results
Phase I: Criteria generation
Phase Ia. ANA as an entry criterion. A systematic review of MEDLINE, EMBASE and the Cochrane database identified 13 080 patients from 64 studies reporting ANA by immunofluorescence on HEp-2 cells. Meta-regression of the operating characteristics of ANA found a sensitivity of 97.8% (95% CI 96.8% to 98.5%) for ANA of ≥1:80 supporting use of ANA as an entry criterion.16 Since some SLE centres do not have access to HEp-2 ANA, and in view of ongoing work on the standardisation of serology and potential future advances in the field, the steering committee and additional autoantibody consultants (MJF and PLM) recommended the provision ‘or an equivalent positive ANA test. Testing by immunofluorescence on HEp-2 cells or a solid phase ANA screening immunoassay with at least equivalent performance is highly recommended’.
Phase Ib. Delphi exercise. One hundred and forty-seven international SLE experts nominated 145 candidate criteria.17 By rating the appropriateness for SLE classification, the participants in the second and third Delphi round reduced the list to 40 candidate criteria (online supplementary table 1).
Supplemental material
Phase Ic. International early SLE cohort. The cohort comprised 616 subjects who had been referred for possible SLE with a disease duration of less than 1 year (n=389 early SLE and n=227 mimicking diseases) from North America, Europe, Asia and South America.18 In addition to supporting many of the 40 candidate criteria derived from the Delphi exercise, the comparison between early SLE and non-SLE patients showed that fever occurred more frequently (34.5% vs 13.7%, p<0.001) in SLE, while SLE patients less commonly suffered from arthralgias (20.3% vs 42.7%, p=0.001) and fatigue (28.3% vs 37%, p=0.02).
Phase Id. Patient survey. 339 SLE patients (>99% Caucasian, 93% female) responded to the survey.19 More than half of these patients reported mucocutaneous findings in the first year of their disease (online supplementary table 1), but also fatigue (89%), joint pain (87%) and fever (54%).19 Given that these items were highlighted both in the early SLE cohort and the patient survey, fever, fatigue and arthralgias were forwarded to the next phase in addition to the 40 Delphi items. Accordingly, phases Ia–Id resulted in a total of 43 candidate criteria for consideration (online supplementary table 1).
Phase II. Criteria reduction
Phase IIa. The expert panel NGT exercise reduced the candidate criteria from 43 to 21.26 The panel distinguished potential ‘entry criteria’, which would be required for classification, from potential ‘additive criteria’. They endorsed ‘positive ANA (≥1:80 by HEp-2 immunofluorescence)’ as an entry criterion. The 20 remaining additive criteria included: lupus nephritis by renal biopsy, autoantibodies, cytopenias, fever, arthritis, serositis, mucocutaneous and neuropsychiatric manifestations (online supplementary table 1).
Phase IIb. Associations between the candidate criteria were evaluated in 389 subjects in the early SLE cohort and the 1000 SLE subjects of the Euro-lupus cohort. Modest statistically significant correlations were limited to the mucocutaneous (r=0.22–0.30), neurological (r=0.22) and immunological (r=0.33) domains in the early SLE cohort, and this modest correlation was replicated in the Euro-lupus cohort.21 Given these associations, criteria were clustered within domains, so that only one criterion within each domain would be counted.
Phase III. Criteria definition and weighting
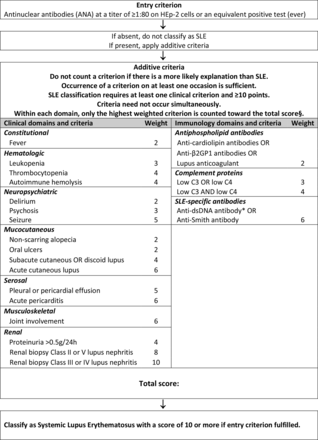
Phase IIIa. Based on the literature, definitions of the 20 candidate additive criteria were refined, using a data-driven evaluation of operating characteristics,22 retaining only feasible items with a prevalence of at least 1% according to literature. Literature-review led to the consensus decision to evaluate five different candidate criteria within the neuropsychiatric domain (delirium, psychosis, seizure, mononeuropathy, cranial neuropathy) and potential separation of acute pericarditis from pleural or pericardial effusions; and between diminished C3 or C4 versus diminished C3 and C4 (online supplementary table 1). The resulting 23 candidate criteria (online supplementary table 1) were organised into seven clinical and three immunological domains, with hierarchical clustering.22 Only the highest-ranking item in each domain was to be counted. Instead of devising exclusion definitions for each criterion, the decision was made to attribute any item to SLE only if no more likely explanation was present. For leucopenia and joint involvement, it was decided to formally test alternative definitions in the derivation cohort. Given the importance of testing for antibodies, particularly for anti-dsDNA, for which tests of relatively low specificity are in use, great care was taken to precisely define testing (table 1).
Definitions of SLE classification criteria
Phase IIIb. The 1.5 day in-person consensus meeting using multicriteria decision analysis involved 74 decisions between pairs of criteria. Criteria weights were calculated by the 1000minds software based on these decisions (table 2). International Society of Nephrology/Renal Pathology Society class III or IV nephritis consistently attained higher weight than class II or V nephritis, so lupus nephritis by histology was separated into two different criteria. Class VI lupus nephritis as an end stage manifestation was unanimously eliminated. Likewise, the experts unanimously voted to not retain mononeuropathy and cranial neuropathy, which had been included into the set of potential neuropsychiatric items in phase IIIa but turned out to add little to SLE classification. The use of weighted criteria led to a sum score that is a measure of the relative probability of a subject having SLE, with higher scores indicating higher likelihood. Experts reached full consensus on a classification of SLE at a provisional threshold score of >83 of a theoretical maximum of 305.24
Relative weights of the additive classification criteria items
Phase IV. Refinement and validation
Twenty-one centres from the USA, Canada, Mexico, Austria, Croatia, France, Germany, Greece, Hungary, Italy, Portugal, Spain, the UK, Turkey, Hong Kong and Japan submitted a total of 2339 cases from their cohorts. 1197 SLE and 1074 non-SLE diagnoses (table 3) were verified by three adjudicators blinded to the proposed classification criteria system. Due to lack of consensus during adjudication, 68 subjects (2.9%) were excluded from the analysis.
Demographic characteristics of the derivation and validation cohorts
Derivation cohort. Of the 2271 triple-adjudicated cases, 501 SLE and 500 non-SLE cases were randomly assigned to the derivation cohort. The provisional weighting system derived from phase III was tested in the derivation cohort. ROC analysis suggested a data-driven threshold of ≥70 (of a maximum of 305), with a sensitivity of 95.4% and a specificity of 95.2%, which was superior to the consensus-derived provisional threshold of >83 that had high specificity (98.8%), but lower sensitivity (81.6%). Review of subjects below the threshold of 70 identified a subgroup of SLE subjects with joint involvement and/or leucopenia. Thus, weights for leucopenia and joint involvement were each adjusted (table 2) to reduce misclassification. When alternative definitions for leucopenia and joint involvement were tested, leucopenia defined as a white blood cell count (WBC) <4.0×10ˆ9/l 3 at least once9 also had a slightly higher sensitivity +specificity (1.944 vs 1.942) than leucopenia defined as WBC <4.0×10ˆ9/l on two or more occasions.6 26 Joint involvement defined as EITHER ‘synovitis involving two or more joints, characterised by swelling or effusion’, OR ‘tenderness in two or more joints and at least 30 min of morning stiffness’9 had a higher combined sensitivity and specificity than arthritis defined simply as synovitis of two or more joints (1.944 vs 1.900). When retested, the revised criteria had increased sensitivity, and maintained sensitivity +specificity. Evaluating ANA as an entry criterion, the criteria with the ANA entry criterion had better performance than without (sensitivity +specificity 1.944 vs 1.930). Next, the weights were simplified by division to whole numbers to achieve a threshold of 10 (table 2). In the derivation cohort, the sensitivity and specificity of the final criteria set (figure 2) were reaching the performance benchmarks set for this project (table 4).
Classification criteria for systemic lupus erythematosus. §Additional criteria items within the same domain will not be counted. *Note: In an assay with at least 90% specificity against relevant disease controls.
Operating characteristics of the new classification criteria compared with the ACR 1997 and SLICC 2012 classification criteria in the derivation and the validation cohorts
Validation. The validation cohort, that is, the full cohort minus the derivation cohort, comprised 1270 triple adjudicated subjects (n=696 SLE, n=574 controls). The criteria, with positive ANA as an entry criterion, weighted criteria in seven clinical domains (constitutional, haematological, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, renal) and three immunological domains (antiphospholipid antibodies, low complements, anti-Smith (anti-Sm) and anti-dsDNA as SLE-specific antibodies) and a classification threshold score of ≥10 (out of a theoretical maximum of 51) (figure 2), had a sensitivity of 96.1% and a specificity of 93.4% (table 4). It demonstrated improved performance compared with the ACR 1997 and SLICC 2012 criteria.
Discussion
New SLE classification criteria were developed with support by both the ACR and EULAR. Through a four-phase, iterative process, we have defined an additive, weighted multicriteria system that produces a measure of the relative probability that an individual can be classified as SLE. The system defines a threshold above which experts would classify cases as SLE for the purpose of research studies. We have carefully defined the criteria to improve reliability and precision; and have grouped the criteria into ten hierarchical domains. We have validated the criteria against a large number of cases, including many patients with manifestations that resemble SLE but who do not have SLE. This approach, as well as the resulting criteria system, represents a paradigm shift for the classification of SLE.
We have defined positive ANA at any time as required entry criterion. There were three possible ways to deal with ANA testing. The previous criteria sets have treated ANA the same as the much more specific antibodies against Sm and dsDNA, which we considered suboptimal given important differences in sensitivity and specificity. We could have excluded ANA completely in classifying lupus, but we still consider ANA a useful test and concept. We therefore decided to test ANA as an entry criterion, which reflects the use of ANA as a highly sensitive screening test.
Criteria using ANA as entry criterion had better performance. During the phase I Delphi exercise, 58% of SLE experts did not feel comfortable and an additional 19% were uncertain about classifying a patient with SLE in the absence of ever having a positive ANA.17 The systematic literature review and meta-regression of data on 13 080 subjects demonstrated ANA ≥1:80 have a sensitivity of 98% with a lower limit of the 95% CI at 97%.16 In the phase I early SLE cohort, 99.5% of the 389 SLE patients were ANA positive.18 The frequencies of ANA positive SLE patients in the derivation and validation cohorts (99.6% and 99.3%, respectively) were in the same range. Since both in the early SLE cohort and in the derivation and validation cohorts, patients were included in many centres worldwide independent of ANA positivity, the latter data provide additional support for ANA as an entry criterion.
Using ANA as entry criterion means the new criteria cannot classify SLE among patients who are persistently ANA negative. While possibly also distinguished by lower cytokine levels27 and lower efficacy of immunomodulatory treatment,28 such a subgroup of patients exists. Although small, it may vary in size in different populations.16 This patient subset needs to be put high on the scientific agenda for further investigation. Additional characterisation of this phenomenon may lead to an alternative entry criterion for this small group of patients. For the moment, we still think it is acceptable to exclude ANA negative patients from clinical trials.
Molecular classification criteria were also considered during the development of these criteria.29 Many novel biomarkers were nominated, such as increased circulating B lymphocyte stimulator (BLyS), IFNγ induced protein 10 kD (IP-10), monocyte chemoattractant protein-1 (MCP-1), TNF-α, type I interferon signature, or increased Th17 and plasma cell populations. They were all voted out in the expert Delphi exercise, largely because of limited availability in the clinical setting and/or insufficient evidence.5 However, inclusion of novel biomarkers, beyond autoantibodies, may ultimately further improve the specificity of SLE classification, increase alignment of classification with underlying disease pathogenesis and improve the performance and information content of clinical trials. Thus, testing of biomarkers against these criteria is an important area for future research.
A new clinical criterion, unexplained fever, turned out to be common and remarkably characteristic for SLE. However, since infections are a major cause of death in SLE, it is of utmost importance to stress that fever, like all other criteria manifestations, should only be counted if no better explanation exists, and that infections have to be suspected first in any patient with (potential) SLE, particularly when CRP is elevated.30 The concept that all criteria are only to be counted if SLE is thought to be the most likely cause of the manifestation (ie, no other more likely cause exists) is central to these new EULAR/ACR criteria, and is explicitly stated as an overarching principle. Some criteria, such as delirium, psychosis and acute pericarditis, were in part redefined based on existing scientific definitions.22 Where alternative definitions were used, the performance of the alternative definitions was comparatively evaluated in the derivation cohort.
The differential weighting of criteria better represents their relative contribution to an individual’s classification of SLE. For SLE, renal biopsy with Class III or IV lupus nephritis carries the most weight and in the presence of a positive ANA is enough to classify a patient as SLE. This further develops a concept of the SLICC criteria9 and reflects the current thinking of SLE experts; in the Delphi exercise, 85% would classify SLE on renal pathology alone.17 Renal biopsy with class II or V lupus nephritis still carries a large weight (eight points) but is not by itself sufficient for the classification of SLE.
The numerical goal of this project was to keep the specificity similar to the specificity of the ACR 1997 criteria, but increase the sensitivity to the high sensitivity level of the SLICC criteria, if possible. The validation cohort data suggest that this goal has been achieved. From our data, it appears that the SLICC criteria increase in sensitivity was to a significant degree founded in accepting renal histology and adding subacute cutaneous lupus and low complement levels. These three advances are mirrored in the current criteria. Many of the other additional symptoms of the SLICC criteria were of very low frequency. Specificity was increased by weighting of criteria, by the NGT expert panel decision to not allow lymphopenia to go forward, and, importantly, by the decision that no criterion be counted if better explained by another condition.
The new criteria provide a simple, directed and highly accurate method for classifying SLE. An electronic ‘app’ is in preparation, which will assist in the use of these criteria. However, it is important to stress that classification criteria are not designed for diagnosis or treatment decisions.5 They should never be used to exclude patients who do not fully meet these criteria from receiving appropriate therapies. This is also pertinent to patients with ANA-negative SLE discussed above. Diagnosis of SLE remains the purview of an appropriately trained physician evaluating an individual patient.5
The new SLE classification system also provides new research opportunities. With much interest in early or latent SLE,31 32 the additive point system and the relative probability of classification it produces, allows for systematic study of individuals who fall below the classification threshold. This will facilitate studies of disease evolution and early intervention. Furthermore, the use of an additive scoring system will allow for studying the idea of ‘ominousity’, that is, the potential implications of having very high scores on disease severity and subsequent prognosis. This work would need to reconsider the relative contribution of individual criteria (weights) and consider additional criteria that potentially contribute to ominousity.
It is anticipated that other groups will test these criteria, which will constitute important external validation. This will be particularly important for paediatric SLE and those with organ dominant, for example, skin dominant disease, since it is a limitation of this criteria project that the patient cohorts do not represent these subgroups. Similar limitations also pertain to several racial/ethnic groups (for example, African American/Black, Hispanic and Asian patients) and to men with SLE, each only included in lower numbers (table 3). It is important to independently test the EULAR/ACR criteria in these subgroups. Leukocyte counts, for example, are more frequently below 4.0×10ˆ9/l in African Americans,33 which may have an influence on criteria performance. It is also possible that the academic center patient populations included differ from patients in community practice clinics. Investigators testing the new criteria in different populations are reminded about the critical importance of the correct attribution of each criterion. Criteria can only be counted when not better explained by another condition. The attribution process requires diligence and clinical experience.
In summary, our multiphase methodological approach and ensuing classification system using ANA as an entry criterion and weighted, hierarchically clustered criteria, constitute a paradigm shift in the classification of SLE. These criteria have excellent performance characteristics and face validity, as the structure and weighting were designed to reflect current thinking about SLE. The inclusion of fever assists with the classification of early SLE. The separation of renal biopsy findings reflects their differential impact on the probability of SLE classification. These criteria have strong operating characteristics, with excellent sensitivity and specificity. This classification system was built using rigorous methodology that was both data-driven and expert-based. With the inclusion of over 200 SLE experts from multiple countries and medical disciplines, methodologists, patient advocates and over 4000 subjects, this work is the largest international, collaborative SLE classification effort to date.
Supplemental material
Acknowledgments
This body of work was jointly supported by the European League Against Rheumatism and the American College of Rheumatology. One part of the derivation and validation cohort was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. The authors wish to acknowledge the diligent work of Banita Aggarwal and Keshini Devakandan in data entry, data cleaning, queries to submitting investigators, data cutting and maintenance of the derivation and validation cohorts; and of Corine Sinnette, MA, in the preparatory work for the multicriteria decision analysis exercise.
References
Supplementary materials
Lay summary
Disclaimer : This is a summary of a scientific article written by a medical professional (“the Original Article”). The Summary is written to assist non medically trained readers to understand general points of the Original Article. It is supplied “as is” without any warranty. You should note that the Original Article (and Summary) may not be fully relevant nor accurate as medical science is constantly changing and errors can occur. It is therefore very important that readers not rely on the content in the Summary and consult their medical professionals for all aspects of their health care and only rely on the Summary if directed to do so by their medical professional. Please view our full Website Terms and Conditions.
Copyright © 2019 BMJ Publishing Group Ltd & European League Against Rheumatism. Medical professionals may print copies for their and their patients and students non commercial use. Other individuals may print a single copy for their personal, non commercial use. For other uses please contact our Rights and Licensing Team.
Footnotes
Permission This article is published simultaneously in the September 2019 issue of Arthritis & Rheumatology.
Handling editor David S Pisetsky
Contributors MA, SRJ, TD, KC, DD, RB, MM, RR-G, JSS, DW, DB, DLK, DJ and RN have been involved in the planning and execution of the project and in writing the manuscript. RC, NC-C, BD, DDG, BH, FH, SJ, DK, KL, EM, W. JM, GR-I, JS-G, MS, MU, GB, BFH, NL, CT, SKT, ZT, GS, BA, FA, TMC, AEC, MKC, LC, AD, WG, BH-K, SH, PMI, MJ,GK, XM, IP, JMP-R, JR-D, IR-F, RS, GHS, YT, MGT, CV, EMV, DJW, SY, PLM and MJF have each significantly contributed to the body of work of the project and involved in correcting and finalising the manuscript.
Funding This study was supported by American College of Rheumatology and European League Against Rheumatism.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Local ethics committees where applicable for chart based review of data.
Provenance and peer review Not commissioned; externally peer reviewed.