Article Text
Abstract
Background The strategy for the choice of the second biologic agent after the failure of the first TNF inhibitor is still an unclear aspect in the treatment of rheumatoid arthritis (RA).
Objectives To evaluatein a real-life settingthe survival on treatment of the second biologic agent in anti-TNF non-responders, comparing patients treated with a second anti-TNF (switch strategy) with patients treated with a different mechanism of action (abatacept, rituximab, or tocilizumab; swap strategy).
Methods We extracted data from a local registry that includes all RA patients treated with biologic therapies between October 1999 and December 2012 in our Rheumatology Unit. We limited our analysis to the period after the first biologic agent other than anti-TNF became available (January 2007-December 2012), in order to minimise differences in exposure among biologics. Patients who stopped a first course TNF inhibitor and started a second line biotherapy since January 2007 were included in the analysis. The rate of drug discontinuation in both subgroups were retrospectively estimated by Kaplan-Meier analysis and compared by log-rank (Mantel-Cox) test.
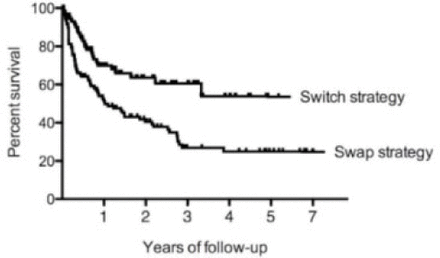
Results Between 1999 and 2012, 641 patients were treated with etanercept (n=192), infliximab (n=221), adalimumab (n=192), golimumab (n=14), or certolizumab pegol (n=22) as first line biotherapy. Since January 2007, 183 of these patients discontinued the TNF blocker because of inefficacy (56%) or safety (44%) issues and switched to a second anti-TNF (n=107) or swapped to abatacept (n=21), rituximab (n=40), or tocilizumab (n=15). The drug survival was significantly greaterin swap thanin switch subgroup(p=0.0009; figure). This difference was confirmed after the stratification of both subgroups according to cause of first anti-TNF discontinuation (inefficacy, p=0.03; safety, p=0.01). No significant differences emerged comparing the retention rate of abatacept, rituximab, and tocilizumab (p=0.2).
Conclusions In a set of clinical practice, the choice of a second biologic agent with different mechanism of action seems to be a better strategy to treat anti-TNF non-responders, irrespective of the cause of TNF blocker discontinuation.
References: Caporali R, et al. Autoimmun Rev 2010; 9(6):465-9.
Disclosure of Interest: None Declared