Article Text
Abstract
Objective: To develop evidence based recommendations for the management of gout.
Methods: The multidisciplinary guideline development group comprised 19 rheumatologists and one evidence based medicine expert representing 13 European countries. Key propositions on management were generated using a Delphi consensus approach. Research evidence was searched systematically for each proposition. Where possible, effect size (ES), number needed to treat, relative risk, odds ratio, and incremental cost-effectiveness ratio were calculated. The quality of evidence was categorised according to the level of evidence. The strength of recommendation (SOR) was assessed using the EULAR visual analogue and ordinal scales.
Results: 12 key propositions were generated after three Delphi rounds. Propositions included both non-pharmacological and pharmacological treatments and addressed symptomatic control of acute gout, urate lowering therapy (ULT), and prophylaxis of acute attacks. The importance of patient education, modification of adverse lifestyle (weight loss if obese; reduced alcohol consumption; low animal purine diet) and treatment of associated comorbidity and risk factors were emphasised. Recommended drugs for acute attacks were oral non-steroidal anti-inflammatory drugs (NSAIDs), oral colchicine (ES = 0.87 (95% confidence interval, 0.25 to 1.50)), or joint aspiration and injection of corticosteroid. ULT is indicated in patients with recurrent acute attacks, arthropathy, tophi, or radiographic changes of gout. Allopurinol was confirmed as effective long term ULT (ES = 1.39 (0.78 to 2.01)). If allopurinol toxicity occurs, options include other xanthine oxidase inhibitors, allopurinol desensitisation, or a uricosuric. The uricosuric benzbromarone is more effective than allopurinol (ES = 1.50 (0.76 to 2.24)) and can be used in patients with mild to moderate renal insufficiency but may be hepatotoxic. When gout is associated with the use of diuretics, the diuretic should be stopped if possible. For prophylaxis against acute attacks, either colchicine 0.5–1 mg daily or an NSAID (with gastroprotection if indicated) are recommended.
Conclusions: 12 key recommendations for management of gout were developed, using a combination of research based evidence and expert consensus. The evidence was evaluated and the SOR provided for each proposition.
- AHS, allopurinol hypersensitivity syndrome
- ES, effect size
- ESCISIT, EULAR Standing Committee for International Clinical Studies Including Therapeutics
- EULAR, European League Against Rheumatism
- ICER, incremental cost-effectiveness ratio
- NNT, number needed to treat
- NSAID, non-steroidal anti-inflammatory drug
- QALY, quality of life years
- RCT, randomised controlled trial
- SOR, strength of recommendation
- SUA, serum uric acid
- VAS, visual analogue scale
- EULAR
- gout
- guidelines
- treatment
Statistics from Altmetric.com
- AHS, allopurinol hypersensitivity syndrome
- ES, effect size
- ESCISIT, EULAR Standing Committee for International Clinical Studies Including Therapeutics
- EULAR, European League Against Rheumatism
- ICER, incremental cost-effectiveness ratio
- NNT, number needed to treat
- NSAID, non-steroidal anti-inflammatory drug
- QALY, quality of life years
- RCT, randomised controlled trial
- SOR, strength of recommendation
- SUA, serum uric acid
- VAS, visual analogue scale
Despite reasonable understanding of its pathogenesis and the availability of effective treatment, gout is often misdiagnosed or diagnosed late in its clinical course, and even when correctly diagnosed treatment is often suboptimal. For example, a recent cross sectional study showed that the prevalence of predefined mismanagement of gout (no drug treatment, analgesic alone, or urate lowering therapy without prophylaxis) was over two times greater with physician management than with patient self management.1 The risk was adjusted by age, sex, education, comorbidity, and number of attacks and was especially high in the first year of disease (relative risk (RR) = 3.8, p<0.005).1 Other medication errors associated with gout appear to be widespread, especially with respect to colchicines.2 Thus the European League Against Rheumatism (EULAR) gout task force was formed to develop evidence based recommendations on aspects relating both to the diagnosis and to the management of gout. This paper reports the second part of the project: evidence based recommendations for the management of gout.
METHODS
Participants
The same multidisciplinary guideline development group as for Diagnosis3 undertook the project. The objectives were, first, to agree key propositions related to the management of gout; second, to identify and critically appraise research evidence for the effectiveness and cost-effectiveness of the relevant treatments; and third, to generate recommendations based on a combination of the best available evidence and expert opinion.
Expert consensus
Up to 10 propositions related to key clinical aspects in the management of gout were formulated, employing the identical Delphi technique and process as that used to develop propositions for Diagnosis.3 However, because the first 10 selected propositions did not address all treatment methods (specifically oral non-steroidal anti-inflammatory drugs (NSAIDs) for acute gout) it was agreed that the final number of propositions should be extended to include the next four propositions with the highest number of votes in the final Delphi round (round 3) and, as with the first 10 propositions, to permit amalgamation or rephrasing if required.
Systematic search of published reports
The same systematic search of reports published between January 1945 and January 2005 was undertaken for both diagnosis and management of gout (for details see part I3 and its appendix 1). Following the Delphi exercise, a proposition specific search, using the same search strategy as for Diagnosis,3 was undertaken.
Inclusion/exclusion criteria
Studies retrieved from the literature search were included only if they were concerned with clinical aspects of gout. Studies of hyperuricaemia were included only if they measured uric acid as an outcome for management of gout. The main focus of interest was on systematic reviews/meta-analyses, randomised controlled trials (RCTs)/controlled trials, uncontrolled trials (for example, one group intervention, quasi-experimental study, and so on), cohort studies, case–control studies, cross sectional studies, and economic evaluations. Case reports, review articles, editorials, and commentaries were excluded. Studies on healthy subjects or animals were also excluded.
Level of evidence
Evidence for efficacy was categorised according to the design characteristics of available studies using an established hierarchy4 (table 1). Questions were answered using the best available evidence. For example, if a question on the effect of an intervention could be answered by level Ia evidence (that is, systematic review of RCTs) then studies of a weaker design (RCT, level Ib) were not reviewed. Results of the latest systematic review were used if there was more than one systematic review for the same question. However, questions on adverse effects were answered using both RCTs and observational studies irrespective of gout, as RCTs are not necessarily the best way to assess adverse effects, and gout may not be the target condition for which the side effects of a particular intervention are assessed. Questions of cost-effectiveness were answered according to the outcome measure of effectiveness. For example, if the effectiveness was measured as “number of attacks prevented” or “quality of life years (QALYs) gained” only studies for gout were eligible. If the effectiveness was measured as “adverse events averted”, any study for the proposed intervention was included.
Level of evidence
Studies with direct evidence were considered first. If no direct evidence was available, studies with indirect evidence were examined. For example, evidence for weight loss in the management of gout was sought first but if none was available evidence for overweight/obesity as a risk factor for gout was examined.
Outcome measures
Efficacy
For treatment efficacy, effect size (ES) compared with placebo or active control as specified within the propositions was calculated for continuous outcomes such as the reduction of serum uric acid (SUA). ES is the standard mean difference—that is, the mean difference between a treatment and a control group divided by the standard deviation of the difference. It is therefore free of units and comparable across interventions. Clinically, an ES of 0.2 is considered small, 0.5 is moderate, and more than 0.8 is large.5 For dichotomous data, such as the percentage of patients with acute attacks or more than 50% pain relief, the number needed to treat (NNT) was estimated.6 The NNT is the estimated number of patients who need to be treated to either prevent an unwanted effect, such as an acute attack, or obtain a wanted outcome such as pain relief; therefore the smaller the NNT the better the treatment effect. The 95% confidence interval (CI) of the NNT was calculated using Altman’s method.7 The dose–response relation between drug treatment and effects was analysed using a linearity test. Individual patient data were obtained from the original reports for this analysis and the results were pooled as appropriate. A multiple regression model was used to adjust co-variables such as concomitant treatment, age, sex, length of the disease, and duration of the treatment.
Adverse effects
For adverse effects, the relative risk (RR) was calculated from RCTs or cohort studies for the incident risk and from cross sectional studies for prevalent risk, whereas the odds ratio (OR) was calculated from case–control studies.8 Both present how many times more likely (or less likely) it is that a subject who is exposed to the drug or intervention will have adverse events than a subject who is not exposed. RR or OR = 1 indicates no increased risk, whereas RR or OR >1 or <1 indicates an increased or decreased risk, respectively.
Economic evaluation
For economic evaluations, the incremental cost-effectiveness ratio (ICER) was calculated as the difference in cost between two treatments divided by their difference in effectiveness. When available, QALYs were used for the measurement of effectiveness; otherwise disease specific outcomes such as the reduction in SUA were used. In addition, study design, comparator, perspective, time horizon, discounting, total costs, and effectiveness were critically appraised.
The outcomes are presented with the point estimate (for example, the mean) and 95% CI unless otherwise stated. Statistical pooling was undertaken as appropriate9 when there was more than one estimate for the same outcome using the same study design and a systematic review was not available.
Ratification of propositions and strength of recommendation
Following the literature search on each proposition and the initial drafting of the manuscript, the task force met to discuss each proposition. At this stage the wording (but not the content) of propositions could be adjusted to clarify specific statements and to reduce any ambiguity if the majority of the task force agreed. Two of the 14 propositions were amalgamated at this stage as they addressed the same intervention topic. The eventual 12 propositions were then ratified and a final adjusted manuscript was approved by all task force members. As for Diagnosis,3 the strength of each recommendation (SOR) was graded using the EULAR A–E ordinal scale (A = fully recommended, B = strongly recommended, C = moderately recommended, D = weakly recommended, and E = not recommended) and a 0–100 mm visual analogue scale (VAS),4 taking into account both the research evidence (efficacy, safety, and cost-effectiveness) and their clinical expertise (logistics, patient perceived acceptance, and tolerability). The mean VAS and 95% CI and the percentage of strongly to fully recommended (A–B) were calculated for each proposition.
Future research agenda
Up to 10 propositions for the future research agenda related to management of gout were formulated, employing the identical Delphi technique and process to that used to develop the future research agenda for Diagnosis.3
RESULTS
General literature search
The general search of published reports yielded 3316 hits (MEDLINE 1111, Old MEDLINE 6, EMBASE 820, CINAHL 17, Science Citation Index 1172, Cochrane 190). After deleting duplications, 2352 remained. Of these, only 181 studies met inclusion criteria, including 83 for diagnosis,3 86 for management, and 12 for both. Figure 1 shows the treatment modalities addressed in the 98 studies related to management; 86% of publications related to pharmacological treatments (for example, NSAIDs and coxibs, colchicine, steroids, allopurinol, febuxostat, uricosuric agents, losartan, fenofibrate); 3% to herbal remedies; and 11% to non-pharmacological treatment (for example, ice, diet). Although a broad range of treatments have been used to manage gout only those agreed using the Delphi consensus approach were assessed. Figure 2 shows the categories of evidence according to study designs for the 98 management related studies.
Treatment methods in the management of gout for which there is published research data. NSAID, non-steroidal anti-inflammatory drug.
Types of evidence in the studies relating to the management of gout. CT, controlled trial; RCT, randomised controlled trial.
Experts’ opinion approach
The experts were informed of the results of the general literature search and then the Delphi exercise was undertaken by email. The first round produced 146 propositions for management. After three anonymous Delphi rounds, 14 propositions were voted in; two of these were amalgamated as they related to the same topic, leaving 12 final propositions (table 2). The wording of eight of these (propositions numbers 1, 2, 3, 5, 7, 9, 11, and 12) were adjusted for clarification of key points at the final meeting.
Propositions and strength of recommendation: order based on topic (general, acute management, and chronic management)
Assessment of propositions
The proposition specific search was then undertaken and the results were merged with the results from the general search to form the basis of evidence for the evaluation of each proposition or methods within each proposition. The propositions are grouped by topic (general, management of acute attacks, urate lowering treatments, prophylaxis of acute attacks) with no weighting according to order.
1. Optimal treatment of gout requires both non-pharmacological and pharmacological modalities and should be tailored according to:
(a) specific risk factors (levels of serum urate, previous attacks, radiographic signs);
(b) clinical phase (acute/recurrent gout, intercritical gout, and chronic tophaceous gout);
(c) general risk factors (age, sex, obesity, alcohol consumption, urate elevating drugs, drug interactions and comorbidity).
Strength of recommendation: 96 (95% CI, 93 to 98)
It is apparent that the management strategy will vary according to the clinical presentation. Asymptomatic hyperuricaemia does not equate to gout and currently there is no evidence to support treatment of isolated hyperuricaemia with urate lowering therapy (ULT), though advice regarding lifestyle and treatment of associated comorbidity may be warranted. Acute gout is extremely painful so a key management objective will be rapid relief of symptoms. By contrast, assessment of a patient during an intercritical period or when chronic tophaceous gout is already present should lead to the development of an individualised long term management plan where the central objective is to reduce tissue levels of uric acid to dissolve existing crystals and to prevent further monosodium urate crystal formation (that is, a “cure”).
The advice given to a patient and the selection and dose of drug treatment will vary according to several factors. For example, the severity of hyperuricaemia and clinical gout, the presence of comorbidity (for example, avoidance of uricosuric drugs in nephrolithiasis; dose adjustment of most drugs with renal impairment and old age), risk factors (weight reduction if obese, reduction in beer and alcohol if excessive), and the patient’s age, sex, and other demographic features. One cohort study compared long term treatment effects (10 years) of urate lowering drugs between patients with chronic gout—grouped according to the presence or absence of tophi or radiographic damage to the affected joints or both—but found no significant difference between groups, as the treatment was effective for all types of patient.10 The dose requirement of allopurinol, used with prophylactic oral colchicine, has been shown to vary between patients in terms of achieving a target SUA level (uncontrolled trials)11,12 and the treatment response varies according to comorbidity such as hypertension and renal impairment (RCTs).13,14
For long term treatment of chronic gout, it has been well documented that either non-pharmacological treatments such as weight loss15 and low purine diet,16 or pharmacological treatments such as allopurinol11–13 are effective. The combination of pharmacological and non-pharmacological treatments (including patient information) appears rational. For example, given that both are effective (table 3), oral colchicine and topical ice packs may be combined to enhance the treatment effect for the relief of pain and other signs of inflammation,17,18 although the two treatments have not yet been investigated in the same RCT using a factorial design. As non-pharmacological treatments are usually less harmful and less costly, they should always be considered either alone or in combination with pharmacological treatments, especially for long term management. With drug treatment, care must be taken to avoid increased toxicity through drug interaction, such as from colchicine with erythromycin (or ciclosporine).19,20
Evidence of efficacy: effect size and number needed to treat
In conclusion, practitioners should always strive for optimal treatment. There is evidence that the combination of non-pharmacological and pharmacological treatments is more effective than individual monotherapy (level Ib). When managing gout it is important to take into account the clinical phase (level Ib), the serum uric acid level and the frequency of previous attacks (level IIb), and associated comorbidity and risk factors (level Ib).
2. Patient education and appropriate lifestyle advice regarding weight loss if obese, diet, and reduced alcohol (especially beer) are core aspects of management.
Strength of recommendation: 95 (95% CI, 91 to 99)
There is a strong belief that patient education and information access is an important determinant of outcome, especially in relation to successful lifestyle alteration and adherence to long term ULT. However, the benefits of education, either alone or as adjuvant therapy, have not been specifically studied in the management of gout.
Two cohort reports have shown that purine-rich food (meat and shellfish) and alcohol consumption (especially beer and spirits) are both associated with gout.27,28 The RR was 1.51 (95% CI, 1.17 to 1.95) for seafood; 1.17 (1.11 to 1.22) for alcohol per 10 g increase; 1.49 (1.32 to 1.70) for beer per serving per day; and 1.15 (1.04 to 1.28) for spirit per serving per day; dairy products were inversely associated with SUA. The risks were independent of other major risk factors such as age, sex, body mass index (BMI), diuretic use, hypertension, and renal failure. However, wine consumption did not increase SUA levels.27,28 A small uncontrolled weight loss trial in 13 patients with gout showed that successful weight loss reduced SUA from 570 μmol/l (95% CI, 520 to 620) at baseline to 470 μmol/l (420 to 520) after 16 weeks of treatment.15 The reduction in SUA occurred earlier (within four weeks) with a specific low purine diet in a larger uncontrolled trial of 305 hyperuricaemic patients.16 As weight loss was also observed in this trial, further studies are required to determine whether diet and weight loss have independent effects.
In conclusion, both low animal purine foods and weight loss reduce SUA in patients with gout (level IIb). Alcohol, particularly beer, is an independent risk factor for gout (level III). Therefore lifestyle advice that addresses obesity, dietary purine intake, and the amount and type of alcohol consumed should be considered in the management of gout. There is general agreement, but no research data, that education on gout and its treatment improves outcome either directly (for example, improved self efficacy) or indirectly through effects on adherence and lifestyle alteration (level IV).
3. Associated comorbidity and risk factors such as hyperlipidaemia, hypertension, hyperglycaemia, obesity and smoking should be addressed as an important part of the management of gout.
Strength of recommendation: 91 (95% CI, 86 to 97)
It is well established that raised SUA is associated with hyperlipidaemia,29–31 hypertension,32,33 diabetes and insulin resistance,34,35 and obesity15,36—conditions that are collectively termed the “metabolic syndrome”. Therefore it would seem obvious good practice to consider these associated conditions when a patient presents with gout. Although there is no direct evidence to support smoking as a risk factor for gout, smoking strongly associates with alcohol consumption,37 which may in turn associate with gout. Importantly, however, smoking is a modifiable risk factor for cardiac and peripheral vascular disease, as well as many other diseases, and therefore needs to be addressed in a holistic approach to patient management.
Apart from the need to detect and treat these co-morbidities in their own right, there is RCT evidence that some of the treatments for these co-morbidities and risk factors may also benefit gout. For example, losartan and fenofibrate both reduce SUA as well as reducing blood pressure and serum lipids, respectively.26,38–43
In conclusion, recognition and treatment of co-morbidities and risk factors should be considered as a part of gout management and global patient care and may benefit both the comorbidity and gout (level Ib).
4. Oral colchicine and/or NSAIDs are first line agents for systemic treatment of acute gout. In the absence of contraindications an NSAID is a convenient and well accepted option.
Strength of recommendation: 94 (95% CI, 91 to 98)
One small (43 patients) and short term (48 hours) open RCT showed that oral colchicine is effective for acute gout.17 This placebo controlled trial examined colchicine at the loading dose of 1 mg followed by 0.5 mg every two hours until development of toxicity (nausea, vomiting, or diarrhoea). The ES was 0.87 (95% CI, 0.25 to 1.50) for pain relief and 1.21 (0.61 to 1.92) for overall clinical improvement. The NNT for at least 50% pain relief was 3 (2 to 11)—that is, one in three patients would experience that degree of pain relief should colchicine be used. However, all 22 patients in the treatment group had nausea, vomiting, or diarrhoea, whereas only five of 21 patients in the placebo group experienced these problems (RR = 4.20 (95% CI, 1.95 to 9.03)) (table 4).
Evidence of safety: relative risk and 95% confidence intervals
Intravenous (IV) colchicine has been used for treating acute gout. However, the potential for severe and even fatal toxicity from this route of administration causes great concern.46,47
NSAIDs have a different mechanism of action but similar symptomatic effects to oral colchicine. One RCT has shown tenoxicam to be more effective than placebo for acute attacks.21 The NNT to obtain more than 50% pain relief was 3 (95% CI, 1 to 14); that is, one in every three patients would achieve more than 50% relief of pain if tenoxicam were used. The results suggest equal efficacy to colchicine although direct comparison between these two agents has yet to be undertaken.
Many head to head comparisons have shown that different NSAIDs give similar benefits in acute gout,24,48–64 with no evidence for individual superiority in terms of clinical efficacy. However, a major concern with NSAIDs is their toxicity on the gastrointestinal tract. Meta-analyses have been undertaken both for evidence of gastrointestinal toxicity and for strategies to minimise gastrointestinal toxicity of NSAIDs, including co-administration of gastrointestinal protectors and alternative use of COX-2 selective inhibitors.4 For acute gout, the COX-2 selective inhibitors rofecoxib and etoricoxib have been investigated.65–67 However, the potential cardiovascular risk from selective and non-selective COX-2 inhibitors has recently been highlighted.68,69 Whether they do more good than harm for gout—a condition which often co-exists with cardiovascular disorders—remains unknown.
In conclusion, oral colchicine or NSAID are both effective at relieving symptoms of acute gout (level Ib). However, colchicine can cause severe diarrhoea, especially in high and frequent dosing, and NSAID use is associated with an increased risk of gastrointestinal bleeding and may have cardiovascular toxicity. Although oral NSAIDs are most commonly used, this preference is largely based on tradition and personal experience as the two treatments have not been directly compared.
5. High doses of colchicine lead to side effects, and low doses (for example 0.5 mg three times daily) may be sufficient for some patients with acute gout.
Strength of recommendation: 83 (95% CI, 74 to 92)
Clinical trials have shown that colchicine at the standard recommended dose (1 g loading dose, followed by 0.5 mg every two to three hours) is effective at relieving symptoms of acute gout.17 It is also effective, at the dose of 0.6 mg three times a day, in preventing acute attacks in patients with chronic gout22 (table 3). However, both dosage regimens cause serious gastrointestinal side effects, especially diarrhoea (table 4). The possibility that a reduced dose or dosing frequency may allow retention of efficacy with a reduction in toxicity is widely debated. However, apart from case reports,70 there is no direct evidence to support a low dose regimen. Studies examining the benefits and risks from different doses of colchicine are still required.
In conclusion, oral colchicine at the high dose schedule is effective but also very toxic, even within a very short treatment period (level Ib). There is popular support for an alternative lower dose regimen, as stated in the proposition, though rigorous evidence to support this new schedule is lacking (level IV).
6. Intra-articular aspiration and injection of a long acting steroid is an effective and safe treatment for an acute attack.
Strength of recommendation: 80 (95% CI, 73 to 87)
Although commonly used in practice, intra-articular aspiration (for immediate reduction of painful intra-articular hypertension as well as for diagnosis) and intra-articular injection of a long acting steroid have not been investigated in controlled trials. In one uncontrolled trial a single intra-articular injection of triamcinolone acetonide 10 mg resulted in pain relief within 48 hours in all 19 patients with acute gout,71 the mean VAS pain score (0–100 mm) reducing from 88 (range 82 to 93) at baseline to 0 (range 0 to 12) at end point. The treatment was well tolerated, no patients had side effects or rebound attacks, and none required additional treatment for the attack. Systemic administration (prednisone, triamcinolone, or ACTH) has also been used in patients in whom an NSAID or colchicine are contraindicated, with reportedly good results.72–76 In practice, this systemic approach is most commonly recommended for patients with severe oligoarticular or polyarticular attacks and for attacks in sites (for example, the midfoot) that are not readily amenable to aspiration. It is generally agreed that neither intra-articular nor systemic steroids should be used if co-existent septic arthritis is suspected.
In conclusion, intra-articular aspiration may be useful for an acute attack but there is no research evidence to support its use (level IV). Intra-articular injection of a long acting steroid is effective at relieving the pain of an acute attack (level IIb). This may be especially useful for patients with a severe mono-articular attack and in those in whom an NSAID and colchicine are contraindicated.
7. Urate lowering therapy is indicated in patients with recurrent acute attacks, arthropathy, tophi, or radiographic changes of gout.
Strength of recommendation: 97 (95% CI, 95 to 99)
Given the unfavourable natural history of untreated gout, non-pharmacological urate lowering treatment (for example, advice on diet, lifestyle modification) should be initiated in every patient at presentation. However, there are sparse research data to guide the decision as to when to start urate lowering drug treatment. There is uniform agreement that urate lowering drugs should be recommended to patients with severe established gout—as indicated, for example, by tophi, gouty arthropathy, radiographic changes of gout, multiple joint involvement, or associated uric acid nephrolithiasis. There is less agreement, however, concerning initiation of urate lowering drug treatment in less severe gout—for example, following clinical presentation with the first acute attack. Opinion ranges from initiation of urate lowering drugs after even the first attack of gout (on the assumption that it is easier to treat and cure gout if there is a relatively small urate crystal load) through to waiting until further attacks occur and become sufficiently frequent to be troublesome (on the assumption that some patients will have relatively infrequent attacks that do not merit long term drug treatment with its associated inconvenience and risk of toxicity). As always, each clinical decision must be individualised according to specific patient characteristics (proposition 1), the balance of risk–benefit of long term drug treatment, and the wishes of the patient. It is agreed that informed patient opinion is central to such decision making (level IV).
8. The therapeutic goal of urate lowering therapy is to promote crystal dissolution and prevent crystal formation. This is achieved by maintaining the serum uric acid below the saturation point for monosodium urate (⩽360 μmol/l or ⩽6 mg/dl).
Strength of recommendation: 91 (95% CI, 86 to 96)
Gout is a true crystal deposition disease which only occurs if urate crystals are present. If further urate crystal formation is halted in a patient and existing crystals are dissolved away, then that patient is essentially “cured”. There are various strategies to reduce tissue urate levels below the saturation point where urate crystal formation can occur and to a low level that encourages crystal dissolution. Apart from urate saturation, the balance of inhibitory and promoting factors in joint tissues also influences urate crystal formation and dissolution; these factors may explain why only a minority of people who are supersaturated for monosodium urate ever form crystals. The level of SUA is presumed to be an indirect indication of joint tissue urate levels. The normal range of SUA is determined by sampling the local population and therefore varies from country to country and with time, depending on the prevalence of factors such as obesity that influence SUA; furthermore the normal range is lower in women, though less so after the menopause. In many populations an SUA that is in the “normal range” may still reflect levels in joint tissues that are above the saturation point for monosodium urate. Thus the target of urate lowering treatment is best centred on an SUA level that is linked to the saturation point of monosodium urate rather than to a normal laboratory range.
A level of SUA of ⩽360 μmol/l reflects a tissue level that is likely to be well below this saturation point. One cohort study has shown that maintaining the SUA below 6.2 mg/dl (370 μmol/l) would significantly reduce tophi, whereas an SUA above 8.2 mg/dl (490 μmol/l) did not reduce tophi.10 This was supported by other two cohort studies in which a linear relation was found between the level of SUA and reduction in tophi,77 and where depletion of urate crystals from knee synovial fluids could be achieved if the SUA was maintained below 6 mg/dl (360 μmol/l) for at least 12 months.78 In some patients—for example, those with extensive tophi and a presumed very high crystal load—the therapeutic target may be to achieve SUA levels that are well below this minimum level to permit a faster “velocity” of tophi reduction.78 The specific SUA level that is made the therapeutic target may thus vary according to individual patient characteristics (proposition 1).
In summary, the aim of urate lowering therapy is “cure” through prevention of urate crystal formation and enhancement of crystal dissolution. To achieve this aim there are clinical data to support the requirement to maintain the SUA at or below a level of 360 μmol/l (6 mg/dl) (level III). This SUA level reflects a tissue level that is below the saturation point for monosodium urate.
9. Allopurinol is an appropriate long term urate lowering therapy. It should be started at a low dose (100 mg daily) and increased by 100 mg every two to four weeks if required. The dose must be adjusted in patients with renal impairment. If allopurinol toxicity occurs, options include other xanthine oxidase inhibitors, a uricosuric agent, or allopurinol desensitisation (the latter only in cases of mild rash).
Strength of recommendation: 91 (95% CI, 88 to 95)
Although allopurinol has been used as an effective treatment for gout for decades, its clinical efficacy has not been examined in placebo controlled RCTs. One open RCT in 59 patients with chronic gout compared the combination of allopurinol 200 mg daily plus colchicine 0.5 mg twice a day (n = 26) against colchicine 0.5 mg twice a day alone (n = 33). After two years a significantly greater reduction in SUA level was observed in those taking allopurinol plus colchicine (ES = 1.39 (95% CI, 0.78 to 2.01)). However, the number of patients experiencing acute attacks was similar in both groups during the first year (NNT = 9 (–9 to 3)); serum creatinine concentrations were also similar in the two groups.25
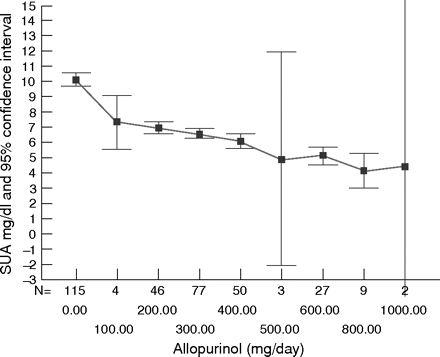
One could question the requirement of a placebo control when a biochemical measure (SUA) is the primary outcome studied. Certainly a large number of uncontrolled trials have shown the urate lowering capability of allopurinol. A re-analysis based on individual patient data from two studies11,12 showed a significant dose–response relation between allopurinol and SUA (fig 3) in which every 100 mg increment of allopurinol reduced SUA by approximately 1 mg/dl (60 μmol/l) (table 5). There is general support for the “go low, go slow” strategy of starting allopurinol at 100 mg daily and increasing by 100 mg increments every few weeks until the therapeutic SUA target is achieved. Compared with giving only a fixed dose of 300 mg (a very common practice throughout Europe), the possible benefits of slowly titrating up the dose include the following: reduced likelihood of provocation of acute attacks; reduced incidence of toxicity; tailoring of the dose to suit individual requirements; and emphasis on the importance of a sufficiently low target SUA. Nevertheless, although this strategy has some face validity and some potential advantages, formal comparison with a fixed dose strategy has not been undertaken.
Effect of reduction of serum uric acid upon treatment variables in patients with primary gout
Allopurinol may cause adverse events including the rare but potentially life threatening allopurinol hypersensitivity syndrome (AHS). This consists of an erythematous desquamating rash, fever, hepatitis, eosinophilia, and worsening renal function.79 One single blind, placebo controlled trial compared renal function (serum creatinine and creatinine clearance) in subjects with hyperuricaemia who received either allopurinol or placebo.13 After 2.5 years of treatment the trial overall found no significant increase of serum creatinine or decrease of creatinine clearance compared with placebo, but there was a reduction in creatinine clearance with allopurinol in hypertensive patients with glomerular filtration rates above 80 ml/min (p<0.02). Unfortunately the trial did not report details of allopurinol dosage and the optimal doses of allopurinol in patients with varying renal function remains unknown, though the principle of using lower (especially starting) doses of allopurinol in patients with impaired renal function is generally accepted. One retrospective cohort study (n = 120) compared the risk of adverse drug reactions between patients whose allopurinol maintenance dose matched the recommended dose according to their creatinine clearance rate (n = 52) and patients whose maintenance dose exceeded the recommended dose (n = 68).44 The risk of rash, AHS, fixed pigmented drug eruption, or leucocytoclastic vasculitis was similar between the two groups (RR = 1.96 (95% CI, 0.34 to 11.92)). Interestingly, one case–control study showed a higher risk of cataract extraction in elderly patients taking allopurinol (OR = 1.82 (1.18 to 2.80)).45
In an economic analysis allopurinol, selected as the prototype and most widely used urate lowering drug, was shown to be more effective (72% v 5% acute attacks averted per year) and more costly than non-urate lowering drug treatment ($426.27 v 267.27 per patient per year).80 ICER (cost per acute attack averted) was $247.40 at base case scenario, and varied from $99.59 to $489.26 depending on patient characteristics and probability estimates. This suggested that for each patient taking allopurinol, it would cost society an additional $99.59 to $489.26 to prevent an additional acute attack of gouty arthritis compared with the strategy of not prescribing urate lowering drugs. Interestingly, the urate lowering drugs become cost saving (that is, more effective and less costly than non-urate lowering drug treatment) once a patient suffers three or more attacks a year.
If allopurinol toxicity does occur, alternative urate lowering treatments may be employed. As discussed in proposition 2, non-pharmacological approaches such as education, weight loss, reducing alcohol consumption, and dietary modification should always be considered. However, if urate lowering drugs are required, the current alternatives are other xanthine oxidase inhibitors or uricosuric agents. Several RCTs have shown that xanthine oxidase inhibitors other than allopurinol (for example, the metabolites of allopurinol, oxipurinol, tisopurine, and febuxostat) are effective in reducing SUA.81–88 However, their safety in patients who previously have developed AHP has not been established. Clinically, up to 40% of patients show cross reactivity between allopurinol and oxipurinol89,90; the non-purine xanthine oxidase febuxostat is not reported to cause severe skin reactions and might be expected to have less cross reactivity than oxipurinol (though this has not been studied directly). Alternatively, as discussed in proposition 10, uricosuric agents may be considered. Allopurinol desensitisation may be successful but is only recommended if the above alternatives fail. It should not be attempted in patients with severe reactions or AHS.91–94
In conclusion, allopurinol is a cost-effective treatment for the long term management of chronic gout (level Ib) and an effective urate lowering drug with a demonstrated dose–response effect on SUA (level IIb). Although not formally studied, the strategy of giving a starting dose of 100 mg daily (especially in those with renal impairment), with further 100 mg increments until the target level of SUA is achieved, is favoured over a fixed dose strategy (level IV). For patients hypersensitive to allopurinol, other urate lowering treatments may be considered. Allopurinol desensitisation is a further option, but only in those with mild hypersensitivity to allopurinol (level IV).
10. Uricosuric agents such as probenecid and sulphinpyrazone can be used as an alternative to allopurinol in patients with normal renal function but are relatively contraindicated in patients with urolithiasis. Benzbromarone can be used in patients with mild to moderate renal insufficiency on a named patient basis but carries a small risk of hepatotoxicity.
Strength of recommendation: 87 (95% CI, 81 to 92)
One controlled trial compared the efficacy of probenecid 1–2 g/day or sulphinpyrazone 400 mg/day to allopurinol 300–600 mg/day for up to two years of treatment 95. Forty patients with uncomplicated chronic gout were allocated to either treatment according to the hospital record number (even or odd). The results showed that a similar number of patients experienced acute attacks (11/20 v 9/17, p = 0.90) but the mean reduction in SUA was greater with allopurinol (4.6 mg/dl or 270 μmol/l) than with probenecid or sulphinpyrazone (3.3 mg/dl or 200 μmol/l). However, detailed statistical data for SUA were not presented. Uncontrolled trials have investigated both agents, one of them showing smaller but statistically significant urate lowering effects of sulphinpyrazone (0.09 mg/dl (95% CI, 0.01 to 0.18)) compared with allopurinol (1 mg/dl (95% CI, 0.94 to 1.24)) per 100 mg incremental dose (table 5). In another uncontrolled trial examined probenecid in patients with and without renal impairment; reduction in SUA was greater in those without renal impairment.96
Benzbromarone was compared with allopurinol in an open RCT of chronic gout patients with renal impairment.14 After two years of treatment the benzbromarone regimen (100–200 mg/day using 50 mg increments until the desired SUA level was achieved) showed a significantly greater reduction of SUA compared with the allopurinol regimen (100–200 mg/day using 50–150 mg increments until the desired SUA level was achieved). The ES was 1.50 (95% CI, 0.76 to 2.24) and more patients achieved the optimal SUA (<6 mg/dl or 360 μmol/l) with benzbromarone (NNT = 3 (95% CI, 2 to 15)). However, because of several case reports of hepatic failure or toxicity,97,98,99,100,101 the use of benzbromarone has become restricted in some European countries.
In conclusion, probenecid and sulphinpyrazone are both effective but probably inferior to allopurinol in lowering SUA (level IIa). They should not be used in patients with renal impairment (level IIb). In contrast, benzbromarone is a powerful uricosuric that is effective, even more so than allopurinol, in patients with renal impairment (level Ib). Its use, however, has been restricted because of rare cases of serious hepatic toxicity.
11. Prophylaxis against acute attacks during the first months of urate lowering therapy can be achieved by colchicine (0.5 to 1 mg daily) and/or an NSAID (with gastro-protection if indicated).
Strength of recommendation: 90 (95% CI, 86 to 95)
Because acute gouty attacks may be induced by the rapid reduction in SUA that follows initiation or an increase in dose of urate lowering drugs,102 strategies have been devised to reduce or prevent such provocation of attacks during the first months of treatment. Two double blind RCTs have examined colchicine in this respect.22,23 In one placebo controlled trial, 43 patients starting allopurinol for gout were randomly allocated to either colchicine 0.6 mg twice daily (n = 21) or placebo (n = 22). After three months, the percentage of patients with acute attacks was significantly less in the treatment group (7/21) than in the placebo group (17/22). The NNT was 2 (95% CI, 1 to 6), suggesting that colchicine would prevent one in two patients from experiencing an attack. However, colchicine also caused more diarrhoea than placebo (RR = 8.38 (95% CI, 1.14 to 61.38)). In a head to head comparison trial, 52 patients with intercritical gout were randomly allocated to probenecid 500 mg three times daily plus colchicine 0.5 mg daily or to probenecid 500 mg three times daily plus placebo daily for six months.23 Both groups showed similar reduction in SUA (ES = −0.44 (95% CI, −1.09 to 0.20)) but the group co-prescribed colchicine had fewer attacks per patient per month than the probenecid-only group (ES = 0.74 (0.08 to 1.40)). Although both groups in this study had similar safety profiles (table 4), the possibility of toxicity, especially neurotoxicity, from long term colchicine treatment requires consideration.
Oral NSAIDs are also used for prophylaxis. Two published controlled trials compared azapropazone (an NSAID with uricosuric effects) 600 mg twice daily with allopurinol,24,49 although one trial49 was part of the other multicentre study.24 Overall, 156 patients were treated for 24 weeks.24 While both treatments showed similar reductions in SUA (ES = 0.00 (95% CI, –0.26 to 0.26)), azapropazone showed additional prophylactic benefit against acute attacks. The NNT was 7 (4 to 17)—that is, treating every seven patients with azapropazone would prevent one more patient from suffering an acute attack than would be the case if allopurinol was used. However, this was offset by a higher incidence of gastrointestinal upset in the azapropazone group (table 4). There are sparse data to guide the duration of prophylaxis; in general, longer prophylaxis is given for patients with greater crystal loads. The benefits of long term prevention must be balanced against toxicity.
In conclusion, evidence to support the use of low dose colchicine for prophylaxis against acute attacks when beginning urate lowering treatment is reasonable (level Ib), whereas evidence for NSAIDs for the same purpose is less convincing (level IIa). Both agents have potentially serious side effects and their benefits and harms need to be carefully weighed.
12. When gout associates with diuretic therapy, stop the diuretic if possible. For hypertension and hyperlipidaemia consider the use of losartan and fenofibrate, respectively (both have modest uricosuric effects).
Strength of recommendation: 88 (95% CI, 82 to 94)
Diuretics, widely prescribed in the community, are a common risk factor for gout (OR = 1.72 (95% CI, 1.67 to 1.76)).103 Depending on its indication, it may be possible to stop chronic diuretic treatment in a patient who develops gout, or switch to an alternative drug regimen that does not contain a diuretic. For patients with gout and hypertension, an antihypertensive regimen that does not contain a thiazide should be considered. The angiotensin II receptor antagonist losartan is not only effective for hypertension but also has a uricosuric action40,41,43; it may therefore lower both blood pressure and SUA.
Apart from hypertension, hyperlipidaemia and other features of the metabolic syndrome are also associated with gout. A double blind, placebo controlled, crossover RCT of the lipid lowering agent fenofibrate has shown uricosuric and serum urate lowering effects.26 Ten patients with hyperlipidaemia were randomly assigned to one of three sequential treatments comprising fenofibrate 100 mg three times daily, bezafibrate 200 mg three times daily, or placebo, also three times daily. Each treatment lasted six weeks, with a three week washout in between. Fenofibrate showed significant reduction of SUA by 20% (95% CI, 14% to 26%) with an effect size of 1.13 (0.18 to 2.07). This reduction was accompanied by a 30% increase in renal uric acid clearance. However, there are no long term randomised controlled studies of losartan or fenofibrate as urate lowering agents for treating gout—either as monotherapy or in combination with other urate lowering drugs—so their clinical value in gout remains unclear.
In conclusion, diuretics should be stopped if possible in patients with gout (level IV) and, if appropriate, alternative antihypertensive treatment without diuretics should be considered (level IV). Uricosuric and urate lowering effects have been shown for the antihypertensive agent losartan (level IIb) and the lipid lowering agent fenofibrate (level Ib), making them attractive for use in gout patients requiring antihypertensive or lipid lowering treatment, respectively. However, the clinical role and cost-effectiveness of these drugs is still unknown.
Future research agenda
Sixty one research topics were recommended initially. The nine that were agreed, after three Delphi rounds, as the most important topics for future research according to currently available research evidence and clinical practice are shown in table 6.
Future research agenda: propositions developed through three Delphi rounds
DISCUSSION
These are the first recommendations for the management of gout to be developed by EULAR. As with previous EULAR task forces for management of specific musculoskeletal disease,4,104–106 we used an evidence based format that permits inclusion of both research evidence and expert opinion while maintaining a clear distinction between the two.
Various other practice guidelines for the management of gout have been published in recent years.107–110 However, the current EULAR recommendations have several differences and possible advantages over these guidelines, including first, an international panel of gout experts permitting broad representation of clinical practice within Europe; second, the inclusion of more recent research data; and third, the use of a rigorous evidence based format. The format that we used involved an anonymous Delphi consensus approach to derive key management propositions; a systematic search for research evidence to support each proposition; the pooling of data across populations where possible; and separate presentation of the category of evidence of supporting research data and the strength of recommendation for each proposition. Possible benefits of such an international evidence based approach include reduction in personal bias, good external validity and generalisability, and ready identification of areas of clinical practice where more research data are required.111 Several methodological issues merit emphasis.
First, we used the EULAR visual analogue and ordinal scale to grade the strength of recommendations.4 Unlike the traditional scale which only reflects the level of efficacy evidence,111,112 the EULAR scales allow a trade off between benefit versus harm, and research evidence versus clinical expertise, and the 95% confidence interval reflects the confidence of the group decision making (the wider the confidence interval the greater the variance within the group in supporting a proposition). This system has been used successfully in other evidence based recommendations4,104,105,113 and is discussed further in the accompanying report on EULAR recommendations for the diagnosis of gout.3
Second, again as discussed in the accompanying report on Diagnosis,3 the task force discussed at length the details relating to the Delphi exercise and the way in which propositions are developed. Particularly pertinent to the management propositions was the decision by the task force to opt for a free range of submitted propositions without specifying specific headings that each needed to be addressed by at least one proposition, and to accept only 10 final propositions, as in previous EULAR projects.4,104–106 The task force realised that this approach would not necessarily result in exhaustive coverage of the topic and indeed, when the preliminary results were presented for feedback at the EULAR congress (Vienna, 2005) there was concern that the first 10 selected propositions did not address all treatment methods (specifically, there was omission of the use of oral NSAIDs for acute gout). Therefore it was agreed that the number of propositions should be extended to include the four with the next highest votes in the final Delphi round (round 3), which then resulted in inclusion of this topic. Nevertheless, these recommendations still only highlight certain aspects of management—they are not designed to be fully comprehensive or to cover every clinical situation related to gout. The task force recommend that for future projects, depending on the disease and the objectives, the possibility be considered of inviting propositions under prespecified headings if comprehensive coverage is desired. Also the more formal inclusion of feedback from EULAR members before finalisation of the recommendations should be considered as this clearly expanded and improved the current recommendations and resulted in a guideline set that more genuinely reflects the views of the EULAR membership. This feedback could be by oral and written communication following presentations at the EULAR Congress, or electronically following display of preliminary recommendations on the EULAR website.
Finally, as with the recommendations for diagnosis, the task force agreed to minor modifications, for the sake of clarity, to the wording of some propositions for management after they had been voted in, researched, and fully discussed, but no change was made to the key content of the propositions at this late stage.
There are various limitations to these recommendations. First, there are caveats relating to the research data. For example, as with any search strategy it is possible that some relevant research data were overlooked; most studies and clinical trials involve specialist referred gout patients who may be unrepresentative of the majority of the population with gout; and the quality of individual studies was not systematically assessed using established check lists such as the CONSORT statement for RCTs or the QUOROM statement for systematic reviews.114,115 Second, although we examined the research evidence and combined this with expert opinion, the third important element of evidence based medicine, patient opinion,116 was omitted. For future task forces ESCISIT is currently considering appropriate ways in which patient opinion can be included. Third, the task force was comprised solely of rheumatologists. The omission of general practitioners, who manage a substantial proportion of gout patients in Europe, may have reduced the generalisability of the recommendations. It was interesting that even within the group of rheumatologists interested in gout there was considerable diversity of practice with respect to certain management issues, most notably when to initiate urate lowering drug treatment in a patient with confirmed gout; whether to use colchicine or NSAID prophylaxis when initiating urate lowering treatment, and what doses to use and for how long; the starting dose of allopurinol, the rate of dose escalation, and the maximum dose that may be used; and the willingness to use intra-articular corticosteroids for an acute attack. Therefore for relevant application of the recommendations we urge the user to study the commentary as well as the statements, to examine the confidence interval for each strength of recommendation (this reflects the diversity of opinion), and to examine the future research agenda which highlights where the group agreed it would be most helpful to have further research data to help guide clinical decisions.
Conclusions
We have developed the first EULAR recommendations for the management of gout based on both clinical practice and the best available evidence. Twelve key recommendations have been evaluated; these include non-pharmacological and pharmacological methods, management of the acute attack of gout, the use of long term urate lowering drug treatment, prophylaxis against acute attacks, and attention to comorbidity. A full review of this topic has also prompted nine key recommendations for the future research agenda. We trust that together with the accompanying propositions for diagnosis3 these recommendations for management will lift the profile of this eminently treatable arthropathy and act as a catalyst for discussion between all health professionals involved in the diagnosis and management of patients with gout.
Acknowledgments
We would like to thank the European League Against Rheumatism for financial support, Helen Richardson for logistic support, Jane Robertson for literature search and database development, and Maggie Wheeler for language translations.
REFERENCES
Footnotes
-
See linked article, p 1301
-
Published Online First 30 May 2006