Article Text
Statistics from Altmetric.com
To direct individual treatment decisions in recent-onset rheumatoid arthritis (RA), predictors of treatment response to methotrexate (MTX) need to be identified. Disease activity at baseline, gender and genetic polymorphisms have already been found to be associated with the effect of MTX treatment, but the predictive value of autoimmune antibody status remains less clear.1 2 It has been shown, however, that both the presence and level of anti-cyclic citrullinated peptide antibodies (ACPA) are strongly associated with a worse disease course.3 Therefore, we investigated the potential predictive effect of levels of ACPA in ACPA-positive patients for the response to MTX treatment. As observations from our cohort and others indicate that ACPA levels decrease during treatment, we studied two selected populations of disease-modifying antirheumatic drug (DMARD)-naïve, ACPA-positive patients with recent-onset arthritis, for whom pretreatment ACPA levels were available.4 5
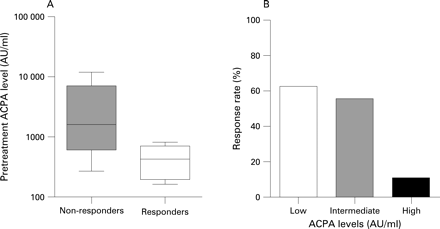
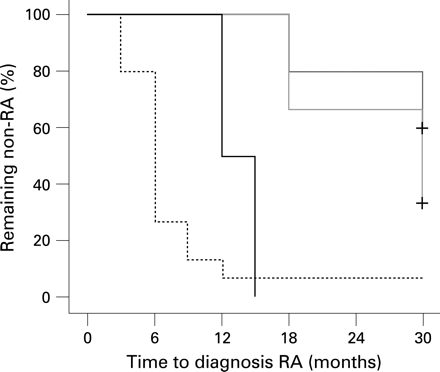
All ACPA-positive patients with undifferentiated arthritis (UA) who were included in the PROMPT study (PRObable RA: Methotrexate versus Placebo Treatment) (MTX group n = 12, placebo group n = 15) were enrolled.5 MTX treatment was started with 15 mg/week and every 3 months the dosage was increased according to the Disease Activity Score (DAS44) to a maximum of 30 mg/week. Responders were defined as patients whose UA did not progress to RA (according to the American College of Rheumatology criteria) during the use of MTX (n = 6/12). MTX responders had lower levels of pretreatment IgG ACPA than non-responders (median (interquartile range) 428 (214–643) AU/ml vs 1594 (781–4495) AU/ml, respectively) (p = 0.024, Mann–Whitney test, fig 1A). Further univariate analysis did not show any significant differences in baseline clinical measures of disease activity, or in rheumatoid factor levels, between responders and non-responders. In addition, the risk of progression to RA, as analysed by survival analysis, was lower in patients with low or intermediate pretreatment ACPA levels than in patients with high levels (stratified by tertiles) (p<0.001, log-rank test, fig 2).
Similar associations were found in a second cohort of ACPA-positive patients with recent-onset RA, who were treated with initial MTX monotherapy (15–25 mg) aiming at a DAS44 ⩽2.4 in the BeSt study and from whom pretreatment serum samples were available (n = 26/131).6 Responders were defined as patients achieving a DAS44 ⩽2.4 after 6 months. The percentage of responders decreased from 63% and 56% in patients with low and intermediate levels, respectively, to 11% in patients with high levels (stratified by tertiles) (p = 0.062, χ2 test, fig 1B). In a multivariate logistic regression analysis, low and intermediate ACPA levels predicted responsiveness, independently of baseline DAS, gender and age (odds ratio = 37, 95% confidence interval 0.8 to 1692, p = 0.064).
Despite the limited number of patients, these data from two distinct cohorts suggest that low and intermediate pretreatment levels of ACPA are associated with a more favourable response to MTX treatment in recent-onset, ACPA-positive arthritis, whereas high levels are associated with an insufficient response. Although these findings have to be confirmed in larger studies, quantitative evaluation of ACPA levels might be an additional tool to determine which patients will benefit most from MTX treatment. Therefore, we propose that pretreatment ACPA levels should be used in future prediction analyses.
Footnotes
Funding: The PROMPT study was financed with grants from the Dutch Arthritis Foundation and the Netherlands Organisation for Scientific Research. The BeSt study was financed with grants from the Dutch College of Health Insurances, Schering-Plough BV and Centocor Inc.
Competing interests: None.
Ethics approval: Ethics committee approval obtained.