Article Text
Abstract
Objectives To assess levels of oxidative DNA damage (8-oxo-7,8-dihydro-2′-deoxyguanine; 8-oxo-dG) and lipid peroxidation (4-hydroxy-2-nonenal; 4-HNE) in serum, synovial fluid and tissue of patients with inflammatory arthritis in relation to in vivo hypoxia levels, disease activity and angiogenic markers.
Methods Oxygen levels in synovial tissue were assessed using an oxygen/temperature probe. Nuclear and cytoplasmic 8-oxo-dG and 4-HNE levels were assessed in synovial tissue from 23 patients by immunohistochemistry. 8-Oxo-dG and 4-HNE levels in serum and synovial fluid were determined using 8-oxo-dG and hexanoyl-Lys (HEL) adduct ELISAs, respectively. Serum vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang2) levels were also measured by ELISA.
Results The median oxygen tension in synovial tissue was profoundly hypoxic at 19.35 mm Hg (2.5%). Nuclear 8-oxo-dG levels were significantly higher than nuclear 4-HNE levels in the lining and sublining layers (all p<0.001). In contrast, cytoplasmic 4-HNE levels were higher than cytoplasmic 8-oxo-dG levels in both cell layers (all p<0.001). Reduced in vivo oxygen tension correlated with high lipid peroxidation in synovial fluid (p=0.027; r=0.54) and tissue (p=0.004; r=0.58). Serum VEGF levels were positively correlated with cytoplasmic 4-HNE expression (p=0.05; r=0.43) and intensity (p=0.006; r=0.59) in the lining layer. Serum Ang2 levels were positively correlated with nuclear 4-HNE expression and intensity in both cell layers (all p≤0.05). DAS28-C-reactive protein was correlated with nuclear 4-HNE expression in the sublining layer (p=0.02; r=0.48) and DAS28-erythrocyte sedimentation rate was correlated with nuclear 4-HNE expression in both cell layers (p≤0.03).
Conclusions Lipid peroxidation is associated with low oxygen tension in vivo, disease activity and angiogenic marker expression in inflammatory arthritis.
Statistics from Altmetric.com
Introduction
Inflammatory arthritis is a chronic progressive disorder associated with joint inflammation, synovial tissue proliferation and degradation of articular cartilage. An early event in inflammation is angiogenesis, where new blood vessels invade the synovial tissue resulting in persistent infiltration of immune cells which causes destruction of adjacent articular cartilage and bone. Although the pathogenesis is unknown and treatment is non-curative, studies suggest that genetic and environmental factors can trigger joint inflammation.1,–,3 Hypoxia and angiogenesis are recognised as important events in the perpetuation of joint destruction in rheumatoid arthritis (RA).4,–,7 Oxygen metabolism and an increase in reactive oxygen species (ROS) also have important roles in the pathogenesis of RA.8 9
Mitochondria are the largest consumers of cellular oxygen and respond to variations in oxygen tension levels which can result in an increased release of ROS, coupled with a compromised antioxidant defence system.10,–,12 Despite multiple conserved redox modulating systems, a proportion of ROS continuously escapes the mitochondrial respiratory chain. The disturbance in the balance between oxidants and antioxidants in favour of the former is defined as oxidative stress and is associated with disease processes including atherosclerosis, chronic inflammation and cancer.13,–,18 While studies have shown that ROS has a role in chronic inflammatory arthritis, this role is not fully understood owing to the highly reactive nature of ROS and the difficulty in detecting levels in vivo.19 Several sources of ROS in the synovial joint have been proposed and chronic oxidative stress of synovial T lymphocytes has recently been found to originate from intracellular ROS production rather than environmental influences.20
Excessive production of ROS can damage biological molecules including DNA, lipids and proteins. Oxidative DNA damage results in formation of DNA adducts such as 8-oxo-7,8-dihydro-2′-deoxyguanine (8-oxo-dG) formed by the reaction of the hydroxyl radical with the DNA guanine base.21 8-Oxo-dG is a pro-mutagenic lesion that mispairs with adenine leading to GC to TA transversion. 4-Hydroxy-2-nonenal (4-HNE) is the main aldehyde formed during lipid peroxidation of 6-polyunsaturated fatty acids by superoxide.22 It exhibits reactivity with proteins, DNA and phospholipids and is an inducer and mediator of oxidative stress.23 It may alter expression of angiogenic factors important in driving disease progression in inflammatory arthritis.24 25
The aim of this study was to determine the levels of oxidative DNA damage and lipid peroxidation in matched serum, synovial fluid and tissue in patients with inflammatory arthritis and to correlate the data with in vivo levels of oxygen tension, disease activity and markers of angiogenesis.
Methods
Patient recruitment
Twenty-three patients with active inflammatory arthritis, rheumatoid and psoriatic arthritis (RA/PsA) were recruited from outpatient clinics at the Department of Rheumatology, St Vincent's University Hospital (16 RA/7 PsA; 10M/13F; median age 53 years, range 30–80). All patients fulfilled the diagnostic criteria for RA26 and PsA27 (median disease duration <54 months since onset of symptoms, range 6–564). Clinical evaluation was performed using standard measures of disease activity including 28 tender and swollen joint counts (DAS28) and modified Health Assessment Questionnaire (HAQ). All samples were taken prior to biological treatment.
Arthroscopy, oxygen tension measurements and sample collection
Under local anaesthetic, arthroscopy of the inflamed knee was performed using a Wolf 2.7 mm needle arthroscope as previously described.28 A LICOX combined oxygen and temperature probe (Integra Life Sciences Corporation, New Jersey, USA) was used to obtain oxygen tension. The oxygen tension and temperature sensitive areas of the probe are 18 mm2 and 23 mm2, respectively. When oxygen diffuses from the tissue through the polyethylene wall into its inner electrolyte chamber, it is transformed into OH− ions at a negatively-charged polarised precious metal electrode, the polarographic cathode, seen as a golden wire end. The current from the oxygen reduction is the raw signal for the sensor. The anode of the circuit is 0.3 cm remote in the rear of the chamber. Initially, the first oxygen tension reading was obtained from the joint cavity, reflecting the oxygen tension in the synovial fluid. After the first synovial biopsy, a sublining tissue pocket was created. Under direct visualisation, the probe was carefully guided and inserted through the outflow port into the pocket, measuring the oxygen tension (mm Hg) and temperature (°C) simultaneously in the synovial tissue. The measurements were taken every 5 min, approximately 20 min in total, until a steady state was achieved with the last value used for analysis.
The biopsy specimen of synovial tissue obtained from the site of the oxygen tension measurement was divided in half; one half was fixed in 10% formalin and paraffin embedded and the other piece was embedded in OCT mounting media and snap frozen in liquid nitrogen. Matching serum and synovial fluid were collected immediately before arthroscopy and stored at −80°C.
8-Oxo-dG and 4-HNE immunohistochemistry
Immunohistochemistry was performed using synovial tissue and the Dako ChemMate Envision Kit (Dako, Glostrup, Denmark). 8-Oxo-dG immunohistochemistry staining was performed using 3 µm paraffin sections and 4-HNE immunohistochemistry staining was performed using 7 µm cryostat sections. Paraffin-embedded sections were baked for 30 min at 90°C, deparaffinised in xylene and rehydrated in alcohol and deionised water. Antigen retrieval was performed by heating sections in antigen retrieval solution (15 ml 1 M sodium citrate and 15 ml 1 M citric acid in deionised water, pH 6.0) in a pressure cooker. The slides were washed in phosphate buffered saline (PBS) and 0.05% Tween for 5 min. Cryostat sections were defrosted at room temperature for 20 min, fixed in acetone for 10 min and washed in PBS for 5 min. The following protocol was identical for paraffin and cryostat sections. Non-specific binding was blocked using 10% casein in PBS for 20 min. 8-Oxo-dG mouse monoclonal antibody and 4-HNE mouse monoclonal antibody (Genox, Baltimore, Maryland, USA) were diluted 1:40 in antibody diluent (Dako) and incubated on sections for 2 h at room temperature in a humidified chamber. An IgG1 control antibody and elimination of the primary antibody were used as negative controls. Endogenous peroxidase activity was blocked using 0.3% hydrogen peroxide for 7 min at room temperature. Slides were washed in PBS and incubated with secondary antibody/HRP (Dako) for 30 min at room temperature. DAB was used to visualise staining, 1:3 Mayer's haematoxylin (BDH Laboratories, Poole, UK) was incubated for 30 s as a DNA counterstain prior to mounting in Pertex mounting media. Images were captured using Olympus DP50 light microscope and AnalySIS software (Soft Imaging System Corporation, Lakewood, Colorado, USA).
Immunohistochemical scoring
8-Oxo-dG and 4-HNE were assessed by two blinded reviewers using an established and validated semiquantitative scoring method.29 30 Both lining and sublining layer cells were assessed for intensity and percentage of nuclear and cytoplasmic staining for both antibodies. Intensity of staining was graded using a scale of 0–3 where 0=negative, 1=weak, 2=moderate and 3=strong. Percentage positivity was graded using a scale of 0–4 where 0=no stained cells, 1=1–25% stained cells, 2=25–50% stained cells, 3=50–75% stained cells and 4=75–100% stained cells.
Hexanoyl-Lys adduct (HEL) and 8-oxo-dG ELISAs
Hexanoyl-Lys adduct (HEL) is a biomarker of lipid peroxidation levels and HEL adducts were assessed in matched serum and synovial fluid samples from arthritic patients (serum, n=20; synovial fluid, n=17). HEL was measured by a competitive ELISA (Gentaur, Belgium) according to the manufacturer's instructions. The microplate was precoated with HEL and the antibody specific for HEL and samples were added to the plate together. Higher concentrations of HEL in the sample resulted in reduced binding of the antibody bound to the surface of the well. Monoclonal antibody bound to the HEL in the sample was washed away and a peroxidase-conjugated secondary antibody was added and bound to anti-HEL antibody. A chromatic substrate was added and absorbance measured at 450 nm. 8-Oxo-dG was measured using a competitive ELISA (Gentaur, Belgium). The microplate was precoated with 8-oxo-dG. 8-Oxo-dG monoclonal primary antibody and samples were added together to the plate. 8-Oxo-dG in the sample and on the precoated plate competes for binding to the monoclonal primary antibody. The monoclonal antibody bound to 8-oxo-dG in the sample was washed and a HRP-labelled secondary antibody was incubated. A chromatic substrate was added; the reaction was then stopped and the absorbance read at 450 nm.
VEGF and Ang2 ELISA
Expression levels of angiogenic factors were assessed in serum samples from arthritic patients (vascular endothelial growth factor (VEGF), n=20; angiopoietin 2 (Ang2), n=18). VEGF and Ang2 were measured by standard sandwich ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, Minnesota, USA).
Statistical analysis
Non-parametric data were assessed using Wilcoxon's signed rank test or Spearman's rank correlation coefficient as appropriate using the Statistical Package for the Social Sciences (SPSS, Chicago, Illinois, USA). All p values are two-sided and p values <0.05 were considered statistically significant in all analyses.
Results
Oxidative DNA damage and lipid peroxidation in synovial tissue
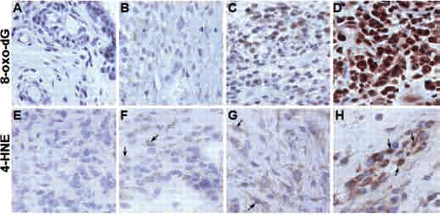
Levels of oxidative DNA damage (8-oxo-dG) and lipid peroxidation (4-HNE) were examined in synovial tissue from 23 patients with inflammatory arthritis. Figure 1A–H shows representative images of 8-oxo-dG (upper panels) and 4-HNE (lower panels) staining in the lining and sublining layers of the synovial tissue. The intensity of staining ranged from negative to weak, moderate and strong. 8-Oxo-dG and 4-HNE were expressed both in the lining and sublining layers of the synovial tissue. Their pattern of staining was both nuclear and cytoplasmic, however 8-oxo-dG staining was predominantly nuclear while 4-HNE staining was mainly cytoplasmic.
(A–H) Representative images of immunohistochemical staining for 8-oxo-7,8-dihydro-2′-deoxyguanine (8-oxo-dG; upper panels) and 4-hydroxy-2-nonenal (4-HNE; lower panels) in the lining and sublining layers of synovial tissue. Intensity of staining was graded from negative (A and E) to weak (B and F), moderate (C and G) and strong (D and H). Arrows in the lower panel indicate intensities of nuclear 4-HNE staining.
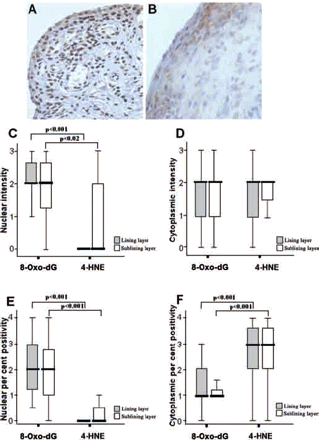
Figure 2A–F shows a comparison of the levels of 8-oxo-dG and 4-HNE in the lining and sublining layers of the synovial tissue. Figures 2A and 2B show representative images of strong nuclear 8-oxo-dG and weak nuclear 4-HNE staining intensity. Figure 2C shows a graphical illustration of intensity levels of nuclear 8-oxo-dG and 4-HNE staining. There is a significantly higher level of nuclear 8-oxo-dG staining intensity compared with nuclear 4-HNE levels in both the lining and sublining layers (p<0.001 and p=0.02, respectively). No difference in cytoplasmic staining intensities was detected for either marker (Figure 2D). The percentage of 8-oxo-dG nuclear positivity was significantly higher compared with 4-HNE nuclear levels in the lining and sublining layers (all p<0.001, Figure 2E). In contrast, the levels of positive cytoplasmic 4-HNE cells were higher than cytoplasmic 8-oxo-dG in both cell layers (all p<0.001, Figure 2F). For all measurements, there was no difference in the levels of oxidative damage and lipid peroxidation between the lining and sublining layers. Figures 3A and 3B show representative images of cytoplasmic 8-oxo-dG staining intensity in patients with RA and PsA, respectively. Figures 3C and 3D show 4-HNE cytoplasmic staining positivity in RA and PsA, respectively. The levels of both markers in RA and PsA were similar (Figures 3E and 3F, respectively).
Comparison of the levels of 8-oxo-7,8-dihydro-2′-deoxyguanine (8-oxo-dG) with 4-hydroxy-2-nonenal (4-HNE) in the lining and sublining layers of paired synovial tissues. (A) and (B) represent strong nuclear 8-oxo-dG intensity staining vs weak 4-HNE staining intensity. Cytoplasmic nuclear 8-oxo-dG and 4-HNE intensity levels were similar. Graphical representations of 8-oxo-dG and 4-HNE (C) nuclear and (D) cytoplasmic intensity levels and (E) nuclear and (F) cytoplasmic percentage positivity in matched synovial tissues using the Wilcoxon signed rank test.
Representative images and levels of 8-oxo-7,8-dihydro-2′-deoxyguanine (8-oxo-dG) and 4-hydroxy-2-nonenal (4-HNE) in patients with rheumatoid arthritis (RA) compared with those with psoriatic arthritis (PsA). The cytoplasmic intensity of 8-oxo-dG is similar in RA and PsA (A, B) and their graphical representation using the Wilcoxon signed rank test is shown in (E). Comparable levels of cytoplasmic expression of 4-HNE in RA and PsA (C, D) and their graphical representation using the Wilcoxon signed rank test is shown in (F).
In vivo oxygen tension measurements in synovial tissue and correlation with oxidative stress
The median oxygen tension in the synovial tissue was 19.35 mm Hg (range 4.3–54), equivalent to an ambient oxygen tension of 2.5% (range 0.42–7%). Oxygen tension levels did not differ significantly between patients with RA and those with PsA (p=0.1; median oxygen tension 26.6 mm Hg for patients with RA and 15.5 mm Hg for patients with PsA). Figures 4A and 4B show representative images of moderate and high levels of cytoplasmic 4-HNE expression in the sublining layer. Tissues with moderate 4-HNE levels had oxygen tension values >20 mm Hg while tissues with high 4-HNE levels had oxygen tension values <20 mm Hg. Figure 4C shows the significant correlation between 4-HNE cytoplasmic expression in the sublining layer and in vivo oxygen tension levels for all patients (Spearman rank correlation: p=0.004; r=0.58). This showed that a high percentage of 4-HNE positive cells was associated with low oxygen tension in the synovial tissue. A similar trend for higher nuclear expression and intensity staining of 4-HNE with reduced in vivo oxygen level was observed, although these correlations did not reach significance. HEL adduct formation was measured in matched synovial fluid and serum samples of patients with inflammatory arthritis. The level of HEL in synovial fluid (n=17) also showed a significant correlation with oxygen tension levels (Figure 4D, Spearman rank correlation: p=0.027; r=0.54). No correlation was detected between serum HEL and oxygen tension levels (Figure 4E). Levels of 8-oxo-dG in serum, synovial fluid and synovial tissue did not correlate with oxygen tension levels.
High lipid peroxidation is associated with a low in vivo oxygen tension (Po2) level in patients with inflammatory arthritis. Representative images of the cytoplasmic percentage of 4-hydroxy-2-nonenal (4-HNE)-positive stained cells in the sublining layer showing (A) moderate staining in patients with Po2 >20 mm Hg and (B) strong staining in patients with Po2 <20 mm Hg. Low oxygen tension in the synovial tissue was significantly correlated with (C) high cytoplasmic expression of 4-HNE in the sublining layer and the level of hexanoyl-Lys (HEL) adduct formation in the synovial fluid (D) but not the serum (E).
Levels of oxidative stress, disease activity and angiogenic marker expression
Levels of lipid peroxidation were correlated with VEGF and Ang2 and disease activity. Table 1 shows a significant positive correlation between serum levels of VEGF and cytoplasmic 4-HNE expression (p=0.05; r=0.43) and intensity (p=0.006; r=0.59) in the lining layer of the synovial tissue. High serum release of Ang2 was associated with nuclear 4-HNE expression (p=0.01; r=0.56) and intensity (p=0.001; r=0.68) in the lining layer and with nuclear 4-HNE expression (p=0.03; r=0.49) and intensity (p=0.05; r=0.43) in the sublining layer of the synovial tissue. To further demonstrate a specific role for oxidative stress in disease progression, microscopic scores plotted against disease activity DAS28-CRP (C-reactive protein) were positively correlated with 4-HNE intensity and expression levels in the lining and sublining layers of the synovial tissue, with significance for nuclear expression in the sublining layer (p=0.02; r=0.48). A positive correlation was also found between DAS28-ESR (erythrocyte sedimentation rate) and 4-HNE nuclear expression in the lining (p=0.03; r=0.49) and sublining layers (p=0.01; r=0.56).
Positive correlations (Spearman rank test) between 4-HNE microscopic scores in the lining and sublining layers of synovial tissue and serum secretion of angiogenic markers and disease activity of patients with inflammatory arthritis
Discussion
This study examined the levels of oxidative DNA damage and lipid peroxidation in patients with inflammatory arthritis. We have shown that increased lipid peroxidation but not DNA oxidative damage is associated with levels of hypoxia in the joint, disease activity and angiogenic marker expression.
Although the cause of chronic inflammation in the joint is unknown, oxidative stress may be important. High levels of oxidative DNA damage, as measured by 8-oxo-dG using high performance liquid chromatography, are detected in mononuclear cells, granulocytes and urine from patients with RA compared with healthy controls.31 32 Levels of 8-oxo-dG in serum and synovial fluid in patients with active RA using competitive ELISA showed similar results.33 In this study we used a specific 8-oxo-dG monoclonal antibody to localise and quantify expression in the lining and sublining layers of the synovial tissue which has not previously been examined. The risk of artefactual production of 8-oxo-dG is associated with DNA extraction and hydrolytic processes; however, this antibody is specific to oxidised DNA and does not recognise RNA.34 As nuclear 8-oxo-dG intensity and expression levels were higher than 4-HNE in the synovial tissue, one might expect a positive association between 8-oxo-dG staining and oxygen tension levels; however, this did not reach significance. Nevertheless, high 8-oxo-dG may still be important in driving disease progression in arthritis.
ROS are lipid peroxidation-inducing agents implicated in inflammatory arthritis and cartilage degradation.35 36 In patients with RA, malondialdehyde, a marker of lipid peroxidation, is negatively correlated with the antioxidant status in plasma.37,–,39 The levels of 4-HNE are significantly higher in the plasma of patients with RA than in those with osteoarthritis (OA).40 In our study we used a monoclonal antibody to assess 4-HNE levels in synovial tissue. This α,β-unsaturated aldehyde is highly reactive and bonds with proteins and DNA and is expressed in many pathological conditions.41 4-HNE can have both antiproliferative and pro-apoptotic effects.42 43 At low levels it has cytoprotective effects and regulates cell proliferation.44 4-HNE levels are higher in osteoblasts from patients with OA than normal cells and high 4-HNE levels are associated with cell death in OA chondrocytes.45 46 However, the role of lipid peroxidation in relation to the oxygen tension in the joint has not previously been described.
In this study, 4-HNE was expressed in the lining and sublining layers and the staining pattern was predominantly cytoplasmic. We found no significant differences in 4-HNE levels in patients with PsA and those with RA, suggesting similar oxidative stress mechanisms in both diseases. Interestingly, we have demonstrated for the first time a significant correlation between cytoplasmic 4-HNE expression, probably reflecting mitochondrial damage in the synovial tissue sublining layer and oxygen tension, where low oxygen tension was associated with a high percentage of 4-HNE positive cells. We also showed that levels of lipid peroxidation in matched synovial fluid (but not serum) significantly correlates with in vivo oxygen tension levels, suggesting that local levels of hypoxia and lipid peroxidation may be an early event, which complements animal studies showing hypoxia at the pre-arthritic stage of the disease.47
As 4-HNE levels correlate with oxygen tension levels in synovial fluid and synovial tissue, we hypothesise that dysfunctional mitochondria alteration induces ROS production which increase 4-HNE. Mitochondrial proteins are targets of 4-HNE adduct formation and 4-HNE can alter mitochondrial uncoupling, transport and pore functions.48,–,51 4-HNE induces cyclooxygenase-2 (COX-2) which controls prostaglandin production during inflammation.52 COX-2 levels are upregulated in inflamed synovial tissue in patients with RA and OA, and COX-2 overexpression with increased prostaglandins results in defects in the mitochondrial respiratory chain.53,–,55 4-HNE stimulates COX-2 expression in macrophages, demonstrating a link between the oxidative modification of lipids and the inflammatory potential of macrophages.56 4-HNE also interferes with the NF-κB signalling pathway, whose activation is associated with inflammation and oxidative stress.57,–,60
ROS mediates angiogenesis and induces endothelial cell proliferation, migration and differentiation.61,–,65 Oxidative stress can increase tubule formation and ets-1 expression which regulates vascular remodelling, cell migration and angiogenesis.66 67 VEGF and hypoxia increase Ang2 expression in microvascular endothelial cells and both VEGF and angiopoietins are required for angiogenesis.68 69 In this study we have shown an association between serum VEGF and Ang2 levels and lipid peroxidation, suggesting that hypoxia and oxidative stress are key regulators influencing VEGF and Ang2 secretion. These may act synergistically in new blood vessel formation.
The definitive role of lipid peroxidation-derived aldehydes in inflammatory arthritis is unknown. A shift in the oxidant/antioxidant balance in favour of lipid peroxidation has been reported, where plasma malondialdehyde was negatively associated with the antioxidant defence system; however, no correlation between malondialdehyde and markers of disease activity in RA has been found.38 70 71 We found that high lipid peroxidation in synovial tissue was positively correlated with clinical disease activity scores. To our knowledge, this is the first report of this correlation. When 4-HNE reacts with proteins they can also become immunogenic.72 Thus, an important consequence of protein modification by 4-HNE may result in the onset of autoimmune reactions or even autoimmune disease processes.73
Overall, these data suggest that levels of lipid peroxidation in patients with inflammatory arthritis are strongly associated with tissue oxygen tension in vivo, disease activity and secretion of angiogenic markers.
Acknowledgments
This work was funded by the Health Research Board of Ireland.
References
Footnotes
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval This study was conducted with the approval of the St Vincent's University Hospital ethics committee.
-
Provenance peer review Not commissioned; externally peer reviewed.