Article Text
Abstract
Objectives: The effect of anti-tumour necrosis factor (TNF) therapy on the antibody responses to vaccines is the subject of ongoing debate. Therefore, we investigated the effect of the three currently available anti-TNF agents on influenza vaccination outcomes in a patient population with long-standing disease.
Methods: In a prospective cohort study, we assessed the antibody response upon influenza vaccination in 112 patients with long-standing autoimmune disease treated with immunosuppressive medication either with anti-TNF (etanercept, adalimumab or infliximab; n = 64) or without anti-TNF (n = 48) and a control group of 18 healthy individuals. Antibody responses were determined by haemagglutination inhibition assay, before and 4 weeks after vaccination.
Results: The proportion of individuals with a protective titre (⩾40) after vaccination was large (80–94%) and did not significantly differ between the three groups. Post-vaccination geometric mean antibody titres against influenza (A/H3N2 and B) were significantly lower in the 64 patients treated with anti-TNF compared with the 48 patients not receiving anti-TNF, and the healthy controls.
Conclusions: The antibody response to influenza vaccination in patients treated with anti-TNF is only modestly impaired. The proportion of patients that achieves a protective titre is not significantly diminished by the use of TNF blocking therapies.
Statistics from Altmetric.com
Anti-tumour necrosis factor-α (anti-TNF) treatment is effective in autoimmune disorders, such as rheumatoid arthritis and Crohn’s disease.1 The three compounds currently registered for clinical use infliximab (Remicade), etanercept (Enbrel) and adalimumab (Humira) differ in the mode of action and incidence rate of opportunistic infections.2 3
Although data on an increased morbidity and mortality due to influenza in patients treated with anti-TNF are lacking, guidelines recommend that patients at risk for complications of influenza, including those treated with anti-TNF, should be annually vaccinated against influenza.4–6 Data on the effect of anti-TNF on the response to influenza vaccination are scarce and conflicting.7–9 The aim of this study is to compare the antibody response upon vaccination against influenza in patients treated with immunosuppressive therapy, including anti-TNF, matched patients not treated with anti-TNF, and healthy controls.
METHODS
Subjects
Patients, 18 years of age or older, treated with anti-TNF at the Leiden University Medical Center, The Netherlands, were invited to participate in this open-label study when attending either the rheumatology or the gastroenterology outpatient clinic. The exclusion criteria were pregnancy, severe chicken egg allergy or an active infectious disease. Approximately 1000 patients with Crohn’s disease and 2000 patients with rheumatoid arthritis visit these outpatient clinics yearly; 207 patients (<7%) were treated with anti-TNF. Sixty-nine (33%) of these patients were enrolled, of whom 64 completed the two study visits (anti-TNF group). Fifty-four patients selected from the same outpatient clinics who were not treated with anti-TNF and 19 healthy controls matched for sex and age were also recruited. Forty-eight patients (no anti-TNF group) and 18 (healthy controls) also completed both study visits. Side-effects or worsening of the underlying condition were not stated as reasons for discontinuing the study by any participant. The protocol was designed according to the good clinical research guidelines and approved by the local medical ethical committee; written informed consent was obtained from all subjects.
Vaccine
All study subjects were vaccinated in the autumn and winter of 2003 with a commercially available trivalent subunit influenza vaccine (Influvac 2003/2004, Solvay Pharmaceuticals BV, Weesp, The Netherlands). The vaccine contained 15 μg of haemagglutinin of each of the following strains: A/Moscow/10/99 (H3N2)-like strain (A/Panama/2007/99 RESVIR-17 reassortant) further referred to as A/H3N2; A/New Caledonia/20/99 (H1N1)-like strain (A/New Caledonia/20/99 IVR-116 reassortant) further referred to as A/H1N1, and B/Hong Kong/330/2001-like strain (B/Shangdong/7/79) further referred to as influenza B. Vaccines were stored at 6°C and administered at room temperature by intramuscular injection in the left deltoid muscle according to the package insert. Patients registered side-effects in a study log.
Antibody assays and statistics
Serum samples were collected at the study visits, at week 0 (before the vaccination) and 4 weeks thereafter and were stored at –80°C until use. The haemagglutination inhibition test was performed in duplicate according to standard methods.10 All sera of each individual study subject were tested simultaneously. For statistical analysis a titre of 5 was arbitrarily assigned to sera with a titre <10. Protection rates were defined as the percentage of patients with a haemagglutination inhibition titre ⩾40 after vaccination, response rates as a fourfold titre increase, in accordance with European guidelines.11 The results were analysed by standard statistical methods: One-way ANOVA for geometric mean titres (GMTs), two-sided χ2 test for protection and response rates. Calculations were performed using SPSS, version 14.0.
RESULTS
One hundred and twelve patients (71% female, median age 49 years, range 18–85) and 18 healthy controls (78% female, median age 52 years, range 21–75) were evaluated (table 1). The patient groups did not significantly differ in age, sex, time on treatment or the use of immunosuppressive drugs (other than anti-TNF) and non-steroidal anti-inflammatory drugs (NSAIDs). The percentage of patients with rheumatic diseases and the percentage of patients who received prior influenza vaccination, in at least one of the 3 years before this study, were significantly higher in the anti-TNF group compared with the control patients. Within the anti-TNF group all three currently available compounds were used, on average for 24 months (range 0.5–78 months): infliximab by 29 (45%; including all patients with inflammatory bowel disease), etanercept by 14 (22%) and adalimumab by 21 (33%).
Four weeks after vaccination against influenza the anti-TNF group had lower GMTs when compared with both the no anti-TNF group and healthy controls. This was significant for two of the three vaccine components (A/H3N2 and influenza B). The no anti-TNF group had insignificantly lower GMTs after vaccination than healthy controls (fig 1). Response rates were significantly lower in the anti-TNF group compared with the no anti-TNF group (p<0.05 for all three antigens). Using slightly different outcome measurements, as used by other authors, did not change our results.7–9
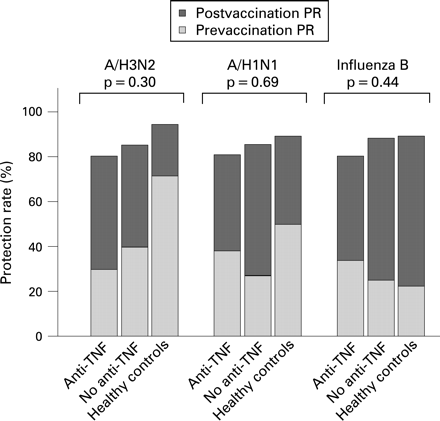
Protection rates 4 weeks after vaccination were high in all groups tested (fig 2). The percentage with protective titres was 89–94% at week 4 in healthy subjects. In both patient groups these percentages were slightly lower, with no statistically significant differences between the three groups.
Infliximab, etanercept and adalimumab, when tested separately, exert similar effects on the response to vaccination. Methotrexate, prednisone, azathioprine and NSAIDs did not influence post-vaccination titres significantly when compared with control groups (data not shown). Linear regression analysis on post-vaccination titres with age, gender, underlying disease, rheumatoid factor positivity, history of previous influenza vaccinations, and pre-vaccination titres as independent variables, showed that only pre-vaccination antibody titres and a history of previous influenza vaccinations significantly contributed to variations in post-vaccination titres. Pre-vaccination titres in healthy controls, resulting from natural infection, were higher compared with both patient groups. After correction the anti-TNF group still had lower post-vaccination titres than the no anti-TNF group.
No major side-effects and no deterioration of the underlying condition were attributed to influenza vaccination. Minor side-effects, such as local pain or tenderness, were frequently reported (20%), with no differences between the three groups. Systemic reactions such as fever, myalgia or headache were reported in 14% of all patients during the 4 weeks after vaccination.
DISCUSSION
The present study demonstrates adequate protection rates against influenza after influenza vaccination in patients treated with anti-TNF, despite lower post-vaccination antibody titres and lower response rates as compared with both similar patients not treated with anti-TNF and healthy controls. To our knowledge this is the first study to include both patients with gastroenterological and rheumatological diseases with longstanding autoimmune diseases requiring treatment, mirroring daily practice. In the present study all three commercially available anti-TNF compounds were used and the average time on this therapy was almost 2 years. In a multivariate analysis, underlying disease (either rheumatological or inflammatory bowel disease), the use of methotrexate or the type of anti-TNF compound used (either infliximab, etanercept or adalimumab) did not influence vaccination outcomes. Up to now, only a few studies have reported on the antibody response upon influenza vaccination in patients treated with anti-TNF.7–9 In line with the current study, these studies agree upon the clinically relevant finding that treatment with anti-TNF does not lower the protection rate (table 2).
The seemingly paradoxical finding of significantly lower post-vaccination titres (and response rates), but equal protection rates, is explained by the fact that the protection threshold (a titre ⩾40) is relatively easily met. Actual post-vaccination titres are therefore considered a better parameter reflecting the immunological competence of a group. This modest, but immunologically relevant, effect was similar for all three antigens and was also consistent in various subgroup analyses (patients with rheumatoid arthritis versus inflammatory bowel disease; patients seronegative before vaccination), although power was lacking to draw firm conclusions concerning these subgroups.
Two other studies report pre- and post-vaccination titres after influenza vaccination in patient groups treated with and without anti-TNF.7 8 In both studies post-vaccination titres were lower in the group treated with anti-TNF as compared with a control group for all three influenza antigens. In the study by Kaine et al patients received only a single dose of adalimumab before vaccination, which might explain the relatively modest effect of anti-TNF on GMTs in this study (table 2).7
Inhibition of TNF, a pivotal cytokine in the type 1 (cell-mediated) immune responses, is most noticeable in T cell mediated reactions. We therefore expected a greater effect of anti-TNF on the T cell dependent responses to the influenza subunit vaccine as compared with the effect on T cell independent responses. In most vaccination studies conducted in patients treated with anti-TNF, the T cell independent pneumococcal polysaccharide vaccine was used and, as expected, none of these studies reported a negative impact of anti-TNF on vaccination outcomes.12–15
In a population consisting of patients with longstanding autoimmune diseases receiving immune-modulating treatment, the proportion of patients with protective titres after a single vaccine dose is substantial (about 80%), irrespective of the immunosuppressive medication or underlying condition. This endorses the current guidelines for a single, annual influenza vaccination of these patients, including those treated with anti-TNF.
Acknowledgments
Influenza vaccines were kindly provided by Solvay-Pharma, Weesp, The Netherlands. We thank Corine Prins, Sandra Numan and Annemiek Versluis, research nurses, LUMC, for their valuable contributions to the collection of materials. We thank Ruud van Beek (research analyst, Erasmus Medical Center, Rotterdam), for his outstanding and swift technical work. J.M. de Jonge-Bok (rheumatologist, Groene Hartziekenhuis, Gouda) and M.L. Westedt (rheumatologist, Bronovo ziekenhuis, Den Haag), who both contributed to patient recruitment.
REFERENCES
Footnotes
Competing interests: None.